Modern Catalysis
The combination of organo-, transition metal and photocatalysis has attracted increasing attention as it can enable the development of unprecedented transformations with high reactivity, efficiency and stereo control.
Counterion-enhanced organocatalysis
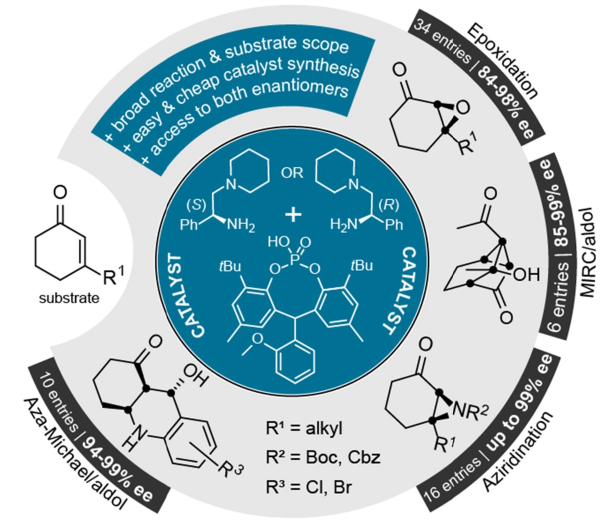
Asymmetric organocatalysis offers an intriguing method of affording optically active compounds without toxic and/or expensive transition metals are required. We are developing novel, natural-derived ion-paired catalysts. The combination of chiral amines and phosphoric acids results in simple, easily accessible, and highly tunable catalyst frameworks. We have already demonstrated this approach to be effective for asymmetric epoxidations, aziridinations, Michael-addition-initiated cascade reactions, asymmetric transfer hydrogenations and asymmetric allylation reactions. Currently, our research focuses on expanding the reaction scope to novel asymmetric transformations, as well as on the combination of asymmetric organocatalysis and photoredox catalysis.
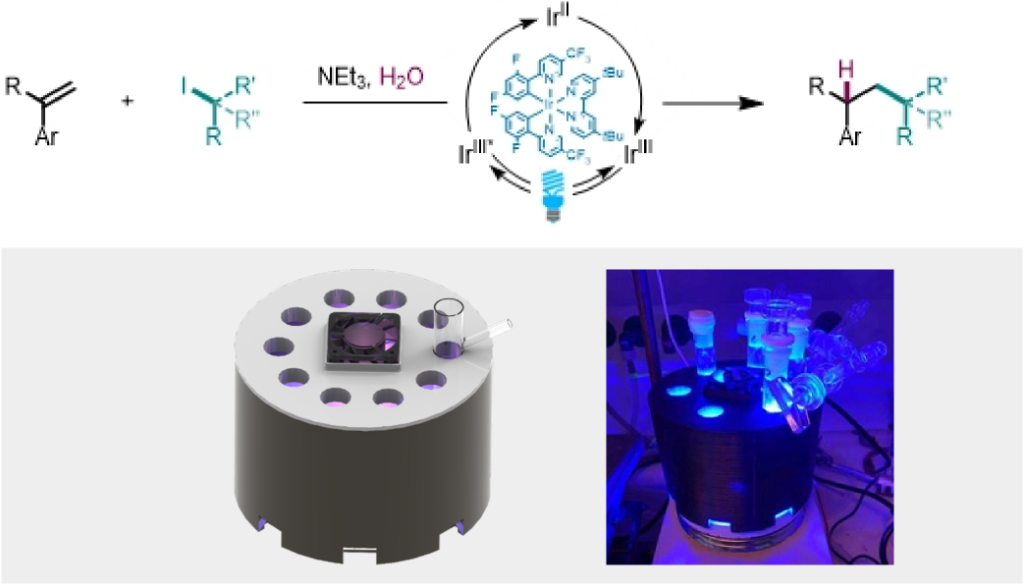
Photocatalysis, visible-light-driven reactions and photo-organocataly
The formation of C-C bonds is one of the most challenging reaction in synthetic organic chemistry. Photocatalysis and light-driven reactions enable complementary reactivities to classical synthetic approaches and therefore, is a powerful tool for exploring novel chemical transformations. We are developing photocatalyst-free, simple light-driven transformations, and aiming to find ways for synthetically challenging problems by merging of classical photoredox and organocatalysis.