
Supported Ionic Liquid Phase (SILP) Allylic Alkylation of Amines in Continuous Flow
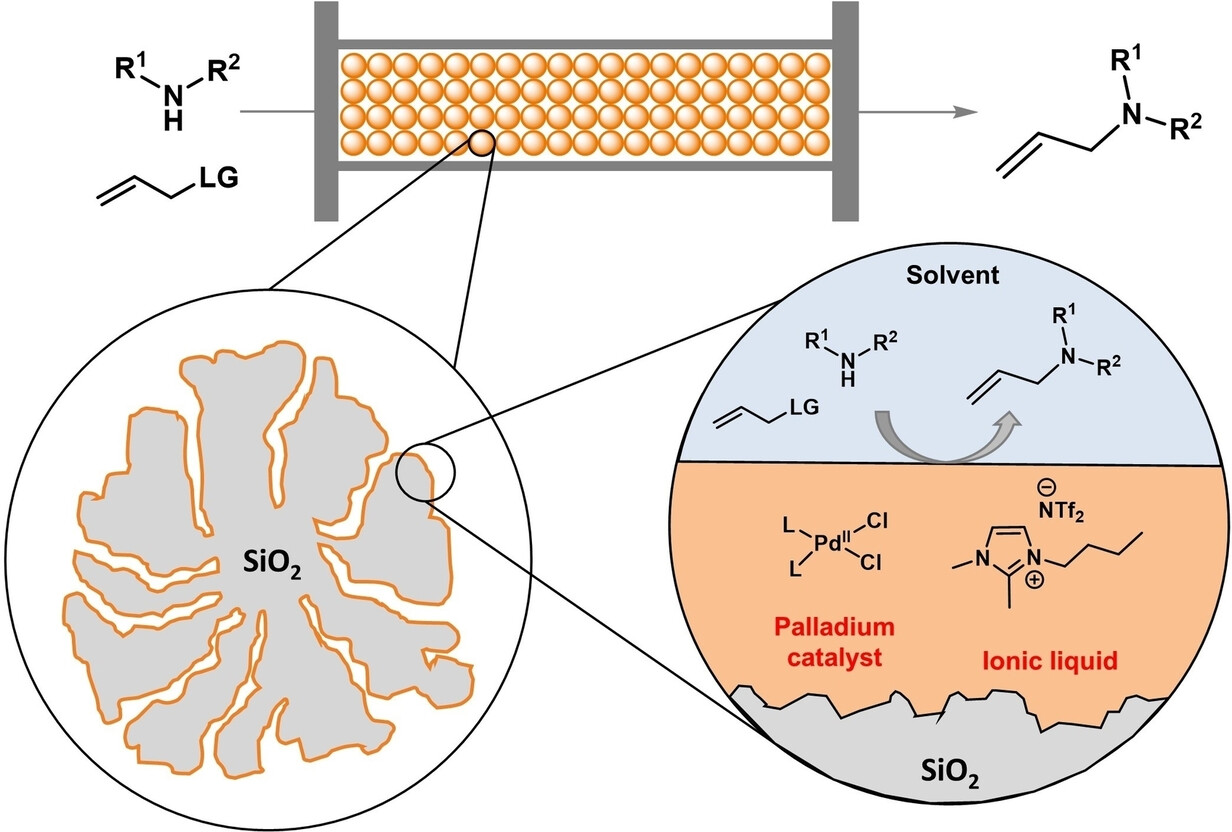
We present the use of Pd-complex-containing supported ionic liquid phases (SILPs) as a novel approach for continuous-flow allylic alkylation of N-nucleophiles. This immobilization strategy gave simple access to air-tolerating catalyst frameworks, providing rapid and convenient access to various achiral and chiral N-allylation products. Under optimized conditions, the flow-reaction could be maintained for 3.5 hours with constant product output; meanwhile, only a marginal 0.7 wt % of ionic liquid leaching and no detectable palladium-complex leaching could be observed.
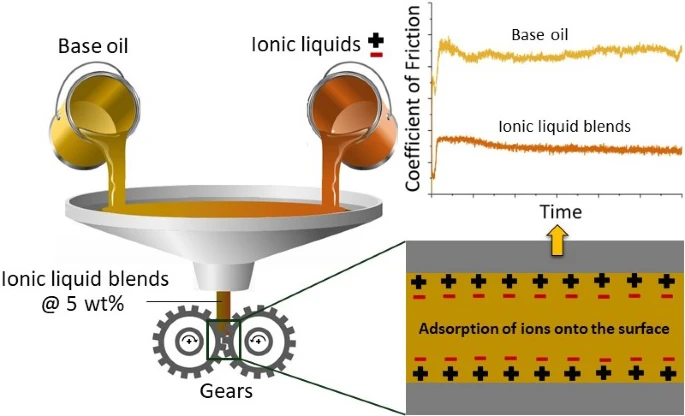
Applying ionic liquids as oil additives for gearboxes: Going beyond the state of the art by bridging the nano-scale and component level
Ionic liquids (ILs) have been used effectively in many applications for reducing problems related to friction and wear. In this work, the potential of ILs as an anti-wear and extreme pressure lubricant additive for high load-carrying gearbox applications such as helicopter transmissions has been studied. Two halide-free ILs: P8881(BuO)2PO−2 (1) and P8881(MeO)2PO−2 (2), which are blended at 5 wt% each into a standard non-additivated FVA2 base oil (BO) are examined. Their solid—liquid interface, friction and load-carrying capacity, and wear (scuffing) behavior are studied on the nano-, lab-, and component-scale, respectively, at a different range of temperature and loading conditions by using the atomic force microscopy (AFM), Schwing—Reib—Verschleiß (SRV) friction tests, and Brugger tests, as well as forschungsstelle für zahnräder und getriebebau (FZG) back-to-back gear test rig. The AFM analysis shows nearly no change of adhesion over the full range of studied temperature for the IL blends compared to the BO. Similarly, IL blends demonstrate a very stable coefficient of friction (COF) of around 0.16, which even decreases with increasing test temperatures ranging from 40 to 120 °C. A clear reduction in COF up to 25% is achieved by adding only 5 wt% of the investigated ILs in the BO, and the Brugger tests also show a pronounced enhancement of load-carrying capacity. Finally, on the component-scale, a significant improvement in gear scuffing performance has been observed for both used IL blends. A detailed characterization of the wear tracks from the SRV friction tests via the transmission electron microscopy (TEM) revealed the formation of a phosphate (P—O)-based amorphous tribo-chemical layer of about 20 nm thickness. Therefore, this work may present an approach for ILs to be used as an additive in conventional lubricants due to their ability to enhance the lubrication properties, making them an alternative lubricant solution for high load-carrying gearbox applications.
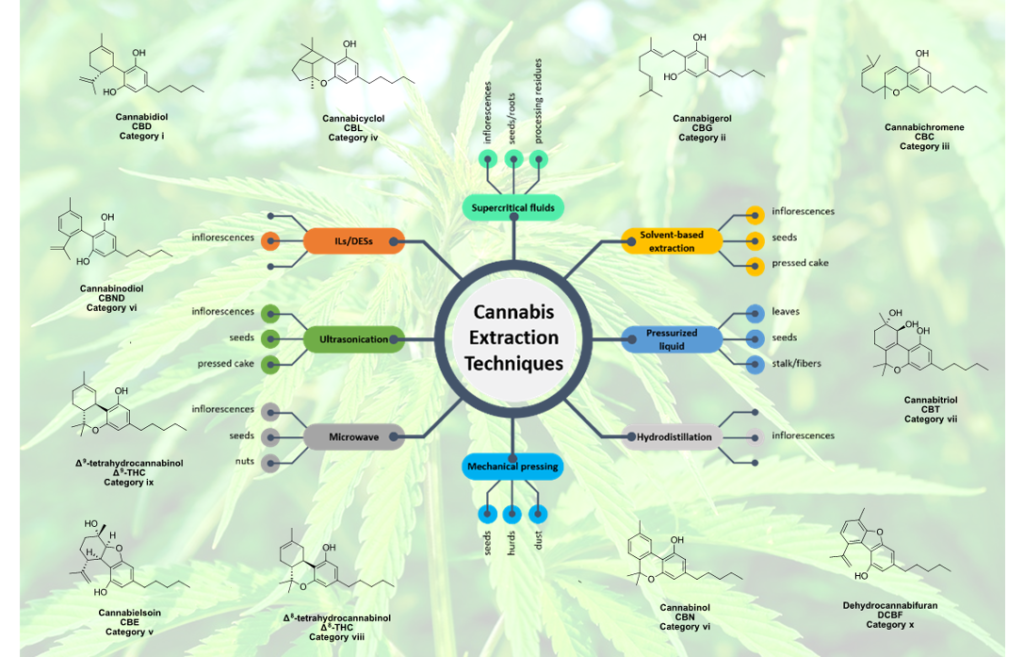
Extraction techniques for bioactive compounds of cannabis
Aitor Sainz Martinez, Olga Lanaridi, Kristof Stagel, Heidi Halbwirth, Michael Schnürch, Katharina Bica-Schröder*
Nat. Prod. Rep. (2023) Advance Article
Historically, cannabis has always constituted a component of the civilized world; archaeological discoveries indicate that it is one of the oldest crops, while, up until the 19th century, cannabis fibers were extensively used in a variety of applications, and its seeds comprised a part of human and livestock nutrition. Additional evidence supports its exploitation for medicinal purposes in the ancient world. The cultivation of cannabis gradually declined as hemp fibers gave way to synthetic fibers, while the intoxicating ability of THC eventually overshadowed the extensive potential of cannabis. Nevertheless, the proven value of certain non-intoxicating cannabinoids, such as CBD and CBN, has recently given rise to an entire market which promotes cannabis-based products. An increase in the research for recovery and exploitation of beneficial cannabinoids has also been observed, with more than 10 000 peer-reviewed research articles published annually. In the present review, a brief overview of the history of cannabis is given. A look into the classification approaches of cannabis plants/species as well as the associated nomenclature is provided, followed by a description of their chemical characteristics and their medically valuable components. The application areas could not be absent from the present review. Still, the main focus of the review is the discussion of work conducted in the field of extraction of valuable bioactive compounds from cannabis. We conclude with a summary of the current status and outlook on the topics that future research should address.
Photocatalyst-free hydroacylations of electron-poor alkenes and enones under visible-light irradiation
Org. Biomol. Chem. (2022) 20, 7245
Herein we present a photocatalyst- and additive-free radical hydroacylation of electron-poor double bonds under mild reaction conditions. Using 4-acyl-Hantzsch ester radical reservoirs, various Michael acceptors, enones and para-quinone methide substrates could be used. The protocol enabled further derivatizations and it could also be extended to a few unactivated alkenes. Moreover, the nature of the radical process was also investigated.

Surface-active ionic liquids: A review
J. Mol. Liq. (2022) 347, 118160
The combination of water and surface-active ionic liquids provides a unique reaction medium, facilitating aggregation and micellization of the ionic liquid to allow for chemical reactions in bulk water. With a growing focus on sustainable technologies, ionic liquids have emerged as tunable solvents for multiple applications but are often too viscous or expensive for use as bulk solvent. As a result, there has been a tremendous increase in interest in the behavior of ionic liquids in the presence of water. It has already been shown that certain ionic liquids act as surfactants in aqueous solutions, enabling the combination of both solvents to afford solvent systems with unique properties. Ultimately, surface-active ionic liquids in water give rise to distinct chemistry of their own compared to traditional molecular solvents, and thus their use is rapidly growing. In this review, the general structure of surface-active ionic liquids and the key features that allow aggregation in water to give micellar structures is discussed. Furthermore, characterization techniques of the formed micelles are presented, discussing aggregation and possible methods of studying micellization behavior. Finally, current applications of surface-active ionic liquids across all fields of chemistry, from traditional organic chemistry to nanoparticle synthesis are presented.

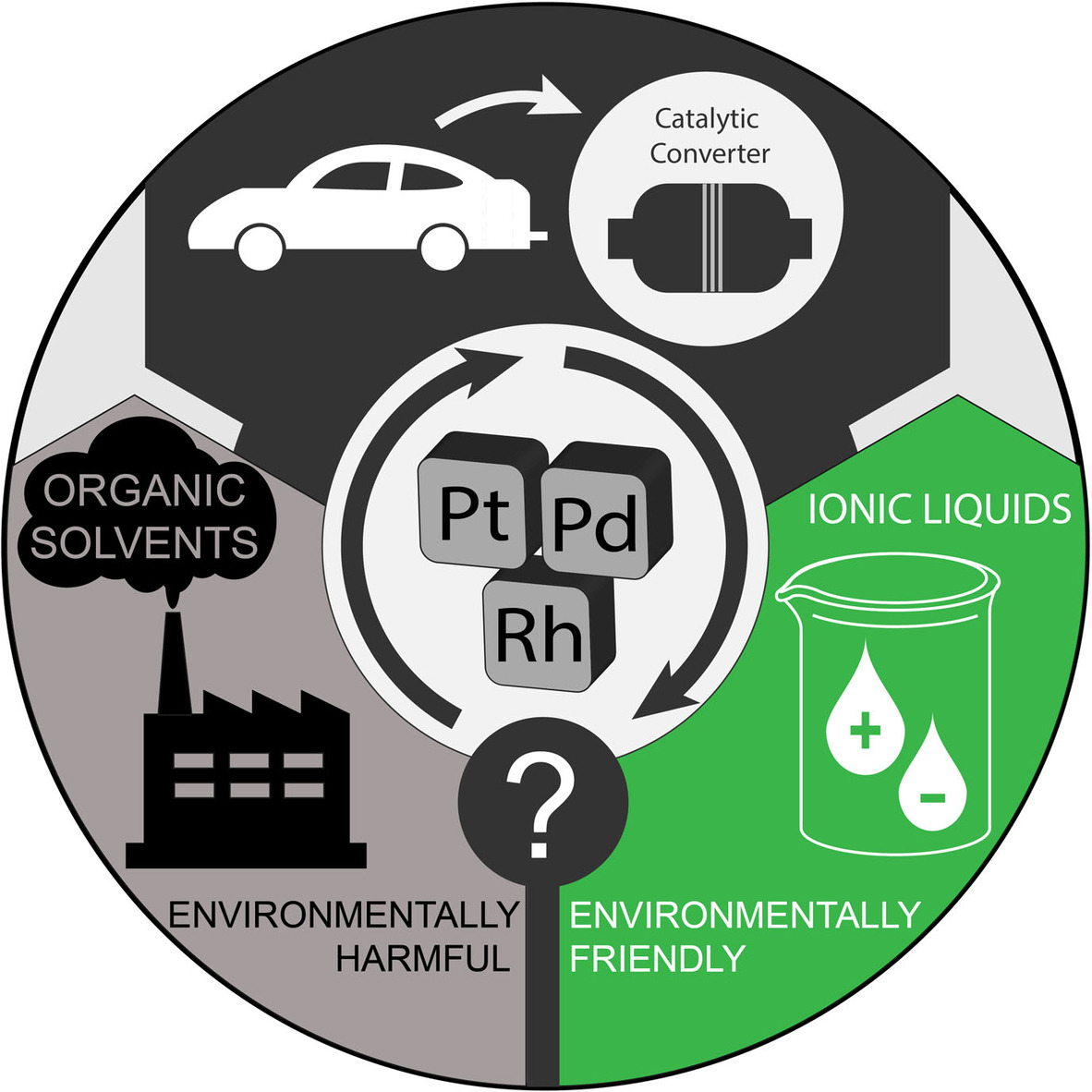
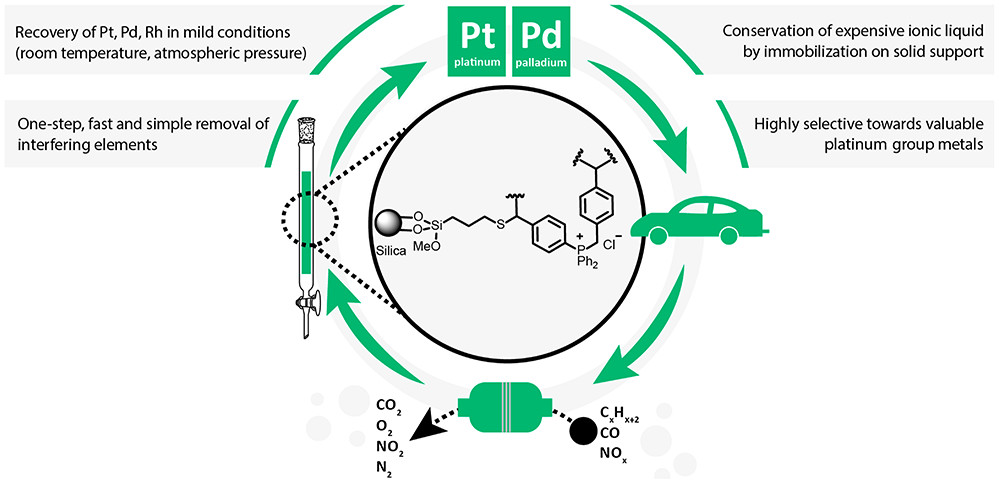
Liquid- and Solid-based Separations Employing Ionic Liquids for the Recovery of Platinum Group Metals Typically Encountered in Catalytic Converters: A Review
ChemSusChem (2022) 15, e202102262
The wide application range and ascending demand for platinum group metals combined with the progressive depletion of their natural resources renders their efficient recycling a very important and pressing matter. Primarily environmental considerations associated with state-of-the-art recovery processes have shifted the focus of the scientific community toward the investigation of alternative recycling approaches. Within this context, ionic liquids have gained considerable attention in the last two decades chiefly sparked by properties such as tunabilty, low-volatility, and relatively easy recyclability. In this review an understanding of the state-of-the-art processes, including their drawbacks and limitations, is provided. The core of the discussion is focused on platinum group metal recovery with ionic liquid-based systems. A brief insight in some environmental considerations related to ionic liquids is also provided while some discussion on research gaps, common misconceptions related to ionic liquids and outlook on unresolved issues could not be absent from this review.
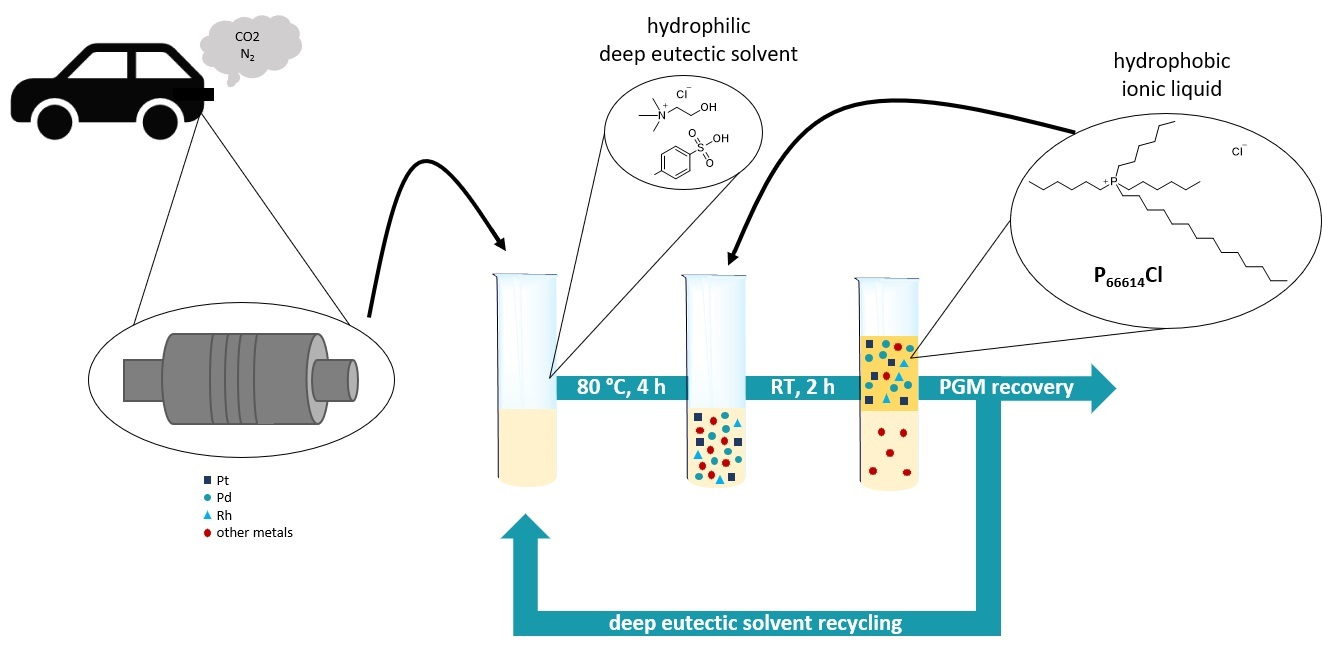
Benign recovery of platinum group metals from spent automotive catalysts using choline-based deep eutectic solvents
Green Chem. Lett. Rev. (2022) 15, 405
The recovery of platinum group metals (PGMs) from secondary raw materials has become a topic ofcritical importance mainly due to the gradual depletion of their natural resources and theircontinuously increasing demand. However, the insufficient recovery of PGMs coupled with thenegative environmental impact of the state-of-the-art recycling procedures mandate theinvestigation and development of alternative recovery processes that will assist in minimizing oreven eliminating these drawbacks. Herein, we present a process for the extraction of platinumgroup metals from spent car catalysts relying on benign deep eutectic solvents (DESs). It isdemonstrated that with addition of small amounts of an oxidizing agent, deep eutectic solventscan act as excellent leaching media for the quantitative extraction of platinum group metals.Despite its inertness towards acidic and oxidizing agents, Rh can be leached in a considerableamount which can be further increased by physical pre-treatment of the spent car catalystmaterial.

Sterically Demanding Flexible Phosphoric Acids for Constructing Efficient and Multi-Purpose Asymmetric Organocatalysts
Angew. Chem. Int. Ed. (2022) 61, e202202189
Herein, we present a novel approach for various asymmetric transformations of cyclic enones. The combination of readily accessible chiral diamines and sterically demanding flexible phosphoric acids resulted in a simple and highly tunable catalyst framework. The careful optimization of the catalyst components led to the identification of a particularly powerful and multi-purpose organocatalyst, which was successfully applied for asymmetric epoxidations, aziridinations, aza-Michael-initiated cyclizations, as well as for a novel Robinson-like Michael-initiated ring closure/aldol cyclization. High catalytic activities and excellent stereocontrol was observed for all four reaction types, indicating the excellent versatility of our catalytic system. Furthermore, a simple change in the diamine’s configuration provided easy access to both product antipodes in all cases.

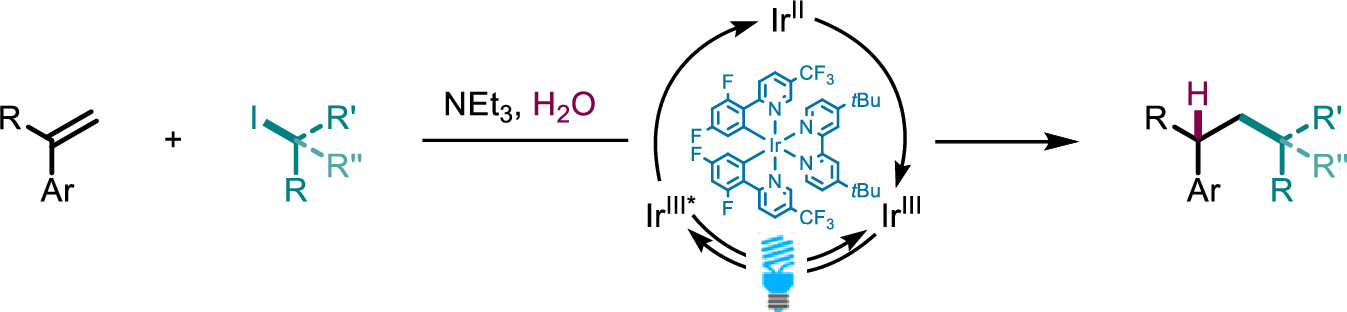
Photocatalytic Hydroalkylation of Aryl-Alkenes
J. Org. Chem. (2022) 87, 11042
Here, we present a visible light-catalyzed hydroalkylation of aryl-alkenes affording C–C bonds using aryl-alkenes and alkyl iodides. We demonstrate the formation of various hydroalkylation products in excellent yields, with primary, secondary, and tertiary alkyl iodides being tolerated in the reaction. Mechanistic experiments reveal a pathway consisting of halogen atom transfer followed by a radical-polar crossover mechanism delivering the desired hydroalkylation products.
Halide-Free Continuous Synthesis of Hydrophobic Ionic Liquids
ACS Sustainable Chem. Eng. (2022) 10, 11215
Herein, we present a novel approach for the halide-free, continuous-flow preparation of hydrophobic ionic liquids (ILs) relying on the bis(trifluoromethanesulfonyl)imide (bistriflimide, NTf2–) anion. The simple yet fast two-step synthetic route, which involves the formation of different alkyl bistriflimides (R4NTf2), followed by the quaternization with an amine nucleophile, led to the desired ILs in high yields and excellent purities without any byproduct formation. The variable alkyl chain (R4) length and the broad range of the applicable nucleophiles (R1R2R3N) offer considerable flexibility to the synthetic protocol. The quaternization can be performed under solvent-free conditions; moreover, the homogeneous nature of these reactions allows the application of modern continuous-flow technologies. Given these advantages, the methodology can afford not just a fast and efficient alternative for the conventional synthesis of such compounds with reduced waste water production but their negligible halide content might provide a significantly broader application range of the IL products, especially for the field of materials science.

Continuous Formation of Limonene Carbonates in Supercritical Carbon Dioxide
Philipp Mikšovsky, Elias N. Horn, Shaghayegh Naghdi, Dominik Eder, Michael Schnürch, Katharina Bica-Schröder*
Org. Process Res. Dev. (2022) 26, 2799
We present a continuous flow method for the conversion of bioderived limonene oxide and limonene dioxide to limonene carbonates using carbon dioxide in its supercritical state as a reagent and sole solvent. Various ammonium- and imidazolium-based ionic liquids were initially investigated in batch mode. For applying the best-performing and selective catalyst tetrabutylammonium chloride in continuous flow, the ionic liquid was physisorbed on mesoporous silica. In addition to the analysis of surface area and pore size distribution of the best-performing supported ionic liquid phase (SILP) catalysts via nitrogen physisorption, SILPs were characterized by diffuse reflectance infrared Fourier transform spectroscopy and thermogravimetric analysis and served as heterogeneous catalysts in continuous flow. Initially, the continuous flow conversion was optimized in short-term experiments resulting in the desired constant product outputs. Under these conditions, the long-term behavior of the SILP system was studied for a period of 48 h; no leaching of catalyst from the supporting material was observed in the case of limonene oxide and resulted in a yield of 16%. For limonene dioxide, just traces of leached catalysts were detected after reducing the catalyst loading from 30 to 15 wt %, thus enabling a constant product output in 17% yield over time.

Counterion-Enhanced Pd/Enamine Catalysis: Direct Asymmetric α-Allylation of Aldehydes with Allylic Alcohols by Chiral Amines and Achiral or Racemic Phosphoric Acids
J. Org. Chem. (2021) 86, 1, 850
We report a straightforward and efficient Pd/enamine catalytic procedure for the direct asymmetric α-allylation of branched aldehydes. The use of simple chiral amines and easily prepared achiral or racemic phosphoric acids, together with a suitable Pd-source resulted in a highly active and enantioselective catalyst system for the allylation of various α-branched aldehydes with different allylic alcohols. The reported procedure could provide an easy access to both product antipodes. Furthermore, two possible orthogonal derivatizations of the enantioenriched aldehydes were performed without any decrease in enantioselectivity.
Toward the Recovery of Platinum Group Metals from a Spent Automotive Catalyst with Supported Ionic Liquid Phases
ACS Sustainable Chem. Eng. (2021) 9, 375
We present a novel approach for the separation and recovery of Pt and Pd leached from a spent automotive catalyst relying on conventional and polymerized supported ionic liquid phases (SILPs and polySILPs, respectively). A variety of parameters with possible effects on the separation behavior, namely, acidity and concentration of the platinum group metal (PGM) containing solution, as well as different SILP and polySILP loadings, were evaluated for the separation of PGMs in the presence of high concentrations of Al, Fe, Zn, and Ce. The polySILP material demonstrated the ability to separate the PGMs from major accompanying interferences in a single separation step, while problems arising from ionic liquid leaching in the case of SILPs could be avoided. Moreover, the use of supported ionic liquid phases allowed the drastic reduction of the amount of required ionic liquid compared to conventional liquid–liquid separation, while avoiding problems arising from emulsion formation. Subsequent stripping experiments lead to further purification of the PGMs and finally desorption from the solid material into a pure solution. Eventually, the concept of chemisorbed polySILPs provides a new and convenient approach for the recycling of platinum group metals.
A Combined Deep Eutectic Solvent–Ionic Liquid Process for the Extraction and Separation of Platinum Group Metals (Pt, Pd, Rh)
Molecules (2021) 26, 7204
Recovery of platinum group metals from spent materials is becoming increasingly relevant due to the high value of these metals and their progressive depletion. In recent years, there is an increased interest in developing alternative and more environmentally benign processes for the recovery of platinum group metals, in line with the increased focus on a sustainable future. To this end, ionic liquids are increasingly investigated as promising candidates that can replace state-of-the-art approaches. Specifically, phosphonium-based ionic liquids have been extensively investigated for the extraction and separation of platinum group metals. In this paper, we present the extraction capacity of several phosphonium-based ionic liquids for platinum group metals from model deep eutectic solvent-based acidic solutions. The most promising candidates, P66614Cl and P66614B2EHP, which exhibited the ability to extract Pt, Pd, and Rh quantitively from a mixed model solution, were additionally evaluated for their capacity to recover these metals from a spent car catalyst previously leached into a choline-based deep eutectic solvent. Specifically, P66614Cl afforded extraction of the three target precious metals from the leachate, while their partial separation from the interfering Al was also achieved since a significant amount (approx. 80%) remained in the leachate.
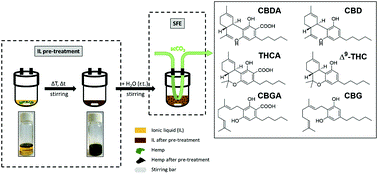
Combined ionic liquid and supercritical carbon dioxide based dynamic extraction of six cannabinoids from Cannabis sativa L.
Green Chem. (2021) 23, 10079
The potential of supercritical CO2 and ionic liquids (ILs) as alternatives to traditional extraction of natural compounds from plant material is of increasing importance. Both techniques offer several advantages over conventional extraction methods. These two alternatives have been separately employed on numerous ocassions, however, until now, they have never been combined for the extraction of secondary metabolites from natural sources, despite properties that complement each other perfectly. Herein, we present the first application of an IL-based dynamic supercritical CO2 extraction of six cannabinoids (CBD, CBDA, Δ9-THC, THCA, CBG and CBGA) from industrial hemp (Cannabis sativa L.). Various process parameters were optimized, i.e., IL-based pre-treatment time and pre-treatment temperature, as well as pressure and temperature during supercritical fluid extraction. In addition, the impact of different ILs on cannabinoid extraction yield was evaluated, namely, 1-ethyl-3-methylimidazolium acetate, choline acetate and 1-ethyl-3-methylimidazolium dimethylphosphate. This novel technique exhibits a synergistic effect that allows the solvent-free acquisition of cannabinoids from industrial hemp, avoiding further processing steps and the additional use of resources. The newly developed IL-based supercritical CO2 extraction results in high yields of the investigated cannabinoids, thus, demonstrating an effective and reliable alternative to established extraction methods. Ultimately, the ILs can be recycled to reduce costs and to improve the sustainability of the developed extraction process.
Dynamic streamlined extraction of iridoids, anthocyanins and lipids from haskap berries
LWT (2021) 138, 110633
The European Commission recently authorized the placing of haskap (Lonicera caerulea L.) berries as a novel food on the EU market. It has been suggested that these berries have several health beneficial properties, due to their considerable content of highly valuable secondary metabolites, such as anthocyanins, phenolic acids, iridoids and fatty acids. Herein, we report a dynamic streamlined extraction process for iridoids and anthocyanins with supercritical carbon dioxide and ethanol as modifier. In particular, we have focused on the iridoids loganic acid and loganin, as well as on different hydroxylation patterns of the anthocyanins. The developed multiple step extraction process leads to initial separation of lipids at low ethanolic concentrations (1 vol%), followed by partial extraction of iridoids at 10 vol% ethanol and ultimately to combined extraction of iridoids and anthocyanins at higher ethanol amounts (>10 vol%). The sequential extraction can be performed in a closed system by automated variation of two innocuous solvents, but uses higher solvent amounts. In comparison to conventional ethanolic extraction, the dynamic streamlined yielded 97% of iridoids and about 7% more anthocyanins.
Purification of anthocyanins from grape pomace by centrifugal partition chromatography
J. Mol. Liq. (2021) 326, 115324
The purpose of this work is to integrate the extraction of anthocyanins from grape pomace with a sequential purification process using an aqueous two-phase system (ATPS), and scaled-up in centrifugal partition chromatography (CPC) using as a common solvent the protic ionic liquids based on ethanolammonium and sulfuric acid. The choice of the best operational condition was performed through the selectivity (ratio between the concentration of anthocyanin and total phenolic compounds), which allowed the purification of anthocyanins of 16.36 fold (S/L = 55 mg.mL−1; 35 °C; [PIL] = 12.5%), and 2.21 fold (S/L = 55 mg.mL−1; 25 °C; [PIL] = 12.5%). ATPS based on protic ionic liquid and acetonitrile purified the anthocyanins between 19.30 and 23.88 fold at 25 °C and pressure atmospheric. At 25 °C (limitation of equipment), CPC increased the purification factor to 41.88, although with the decrease of anthocyanin recovery. However, the best operational condition depends on the anthocyanins application.
Chemical composition and antioxidant potential of Cannabis sativa L. roots
Christoph Kornpointner, Aitor Sainz Martinez, Silvija Marinovic, Christian Haselmair-Gosch, Polona Jamnik, Katharina Schröder, Christian Löfke, Heidi Halbwirth*
Ind. Crops Prod. (2021) 165, 113422
Cannabis sativa L. has long been exploited for multiple purposes. Whereas all parts of the shoots are extensively used and well investigated, the roots have always received less attention. The phytochemical spectrum of the roots differs significantly from the rest of the plant, as no significant amounts of cannabinoids are found, whereas triterpenes as well as phytosterols are abundantly present. To shed light on the unique phytochemistry of hemp roots and the related industrial potential, three chemovars were investigated for the secondary metabolite composition and antioxidant activities by using in vitro and in vivo methods. Five triterpenes, ten phytosterols and five aliphatic compounds were identified by GC–MS analysis. Glutinol, ß-amyrone, stigmastanol, fucosterol, stigmasta-3,5-diene, stigmasta-3,5,22-triene, and oleamide were described for the first time in cannabis root extracts. The predominant triterpenoids friedelin (0.100−0.709 mg/g) and epifriedelinol (0.059−0.205 mg/g) were quantified in dependence of chemovar, harvest times, drying conditions, and extraction efficiency with ethanol, n-hexane, and supercritical CO2.
Chiral Phosphoric Acids as Versatile Tools for Organocatalytic Asymmetric Transfer Hydrogenations
Ádám Márk Pálvölgyi, Fabian Scharinger, Michael Schnürch, Katharina Bica-Schröder*
Eur. J. Org. Chem. (2021) 2021, 5367
Herein, recent developments in the field of organocatalytic asymmetric transfer hydrogenation (ATH) of C=N, C=O and C=C double bonds using chiral phosphoric acid catalysis are reviewed. This still rapidly growing area of asymmetric catalysis relies on metal-free catalysts in combination with biomimetic hydrogen sources. Chiral phosphoric acids have proven to be extremely versatile tools in this area, providing highly active and enantioselective alternatives for the asymmetric reduction of α,β-unsaturated carbonyl compounds, imines and various heterocycles. Eventually, such transformations are more and more often used in multicomponent/cascade reactions, which undoubtedly shows their great synthetic potential and the bright future of organocatalytic asymmetric transfer hydrogenations.
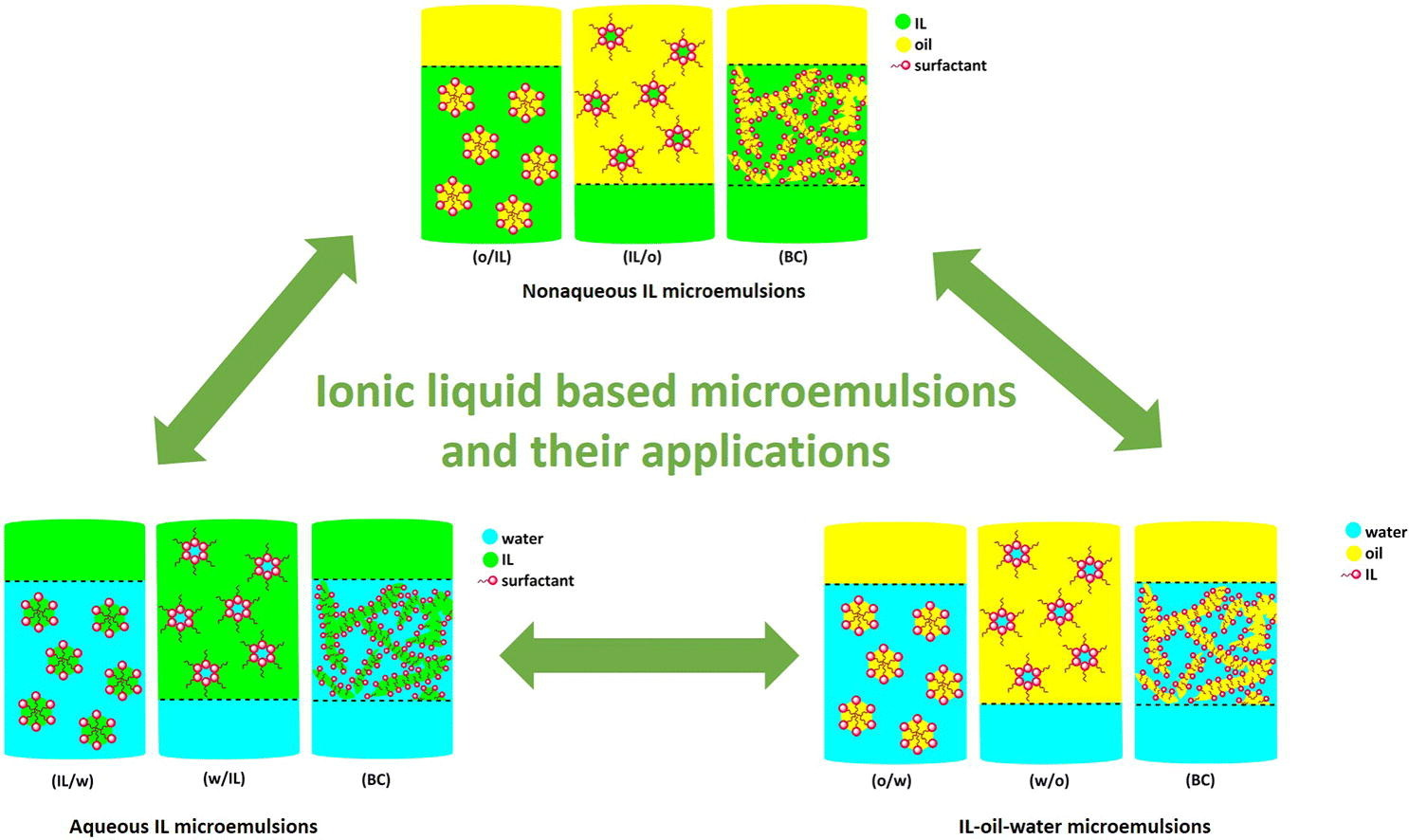
Ionic liquid based microemulsions: A review
J. Mol. Liq. (2020) 303, 112264
Over the past few years an increasing number of studies dealt with microemulsions, in which either the aqueous phase, the oil phase, the surfactant or even two of these components constitute of an ionic liquid. Many examples demonstrated that water and oil are not necessarily the required polar and non-polar components that contribute to the formation of microemulsions but can be replaced by targeted ionic liquids. Moreover, in many cases ionic liquids are used as amphiphiles that assist in the formation of microemulsions in aqueous or non-aqueous media due to their unique surface activity.
In the present review, we highlight the properties of three types of ionic liquid based microemulsions, namely non-aqueous ionic liquid microemulsions, aqueous ionic liquid microemulsions and ionic liquid/oil/water microemulsions. Various characterization techniques applied for the confirmation of their formation and the detailed elucidation of their properties are discussed herein. Additionally, we present a comprehensive summary of the recent applications of ionic liquid based microemulsions in different fields, such as synthesis, (bio‑)catalysis, polymerization, (nano‑)material preparation, drug delivery and separations.
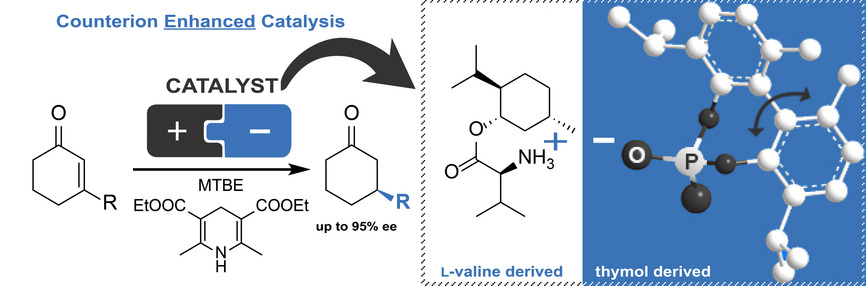
Counterion Enhanced Organocatalysis: A Novel Approach for the Asymmetric Transfer Hydrogenation of Enones
ChemCatChem (2020) 12, 3776
We present a novel strategy for organocatalytic transfer hydrogenations relying on an ion-paired catalyst of natural L-amino acids as main source of chirality in combination with racemic, atropisomeric phosphoric acids as counteranion. The combination of a chiral cation with a structurally flexible anion resulted in a novel chiral framework for asymmetric transfer hydrogenations with enhanced selectivity through synergistic effects. The optimized catalytic system, in combination with a Hantzsch ester as hydrogen source for biomimetic transfer hydrogenation, enabled high enantioselectivity and excellent yields for a series of α,β-unsaturated cyclohexenones under mild conditions. Moreover, owing to the use of readily available and chiral pool-derived building blocks, it could be prepared in a straightforward and significantly cheaper way compared to the current state of the art.
Enantiomerization of Axially Chiral Biphenyls: Polarizable MD Simulations in Water and Butylmethylether
Int. J. Mol. Sci. (2020) 21, 6222
In this study, we investigate the influence of chiral and achiral cations on the enantiomerization of biphenylic anions in n-butylmethylether and water. In addition to the impact of the cations and solvent molecules on the free energy profile of rotation, we also explore if chirality transfer between a chiral cation and the biphenylic anion is possible, i.e., if pairing with a chiral cation can energetically favour one conformer of the anion via diastereomeric complex formation. The quantum-mechanical calculations are accompanied by polarizable MD simulations using umbrella sampling to study the impact of solvents of different polarity in more detail. We also discuss how accurate polarizable force fields for biphenylic anions can be constructed from quantum-mechanical reference data.
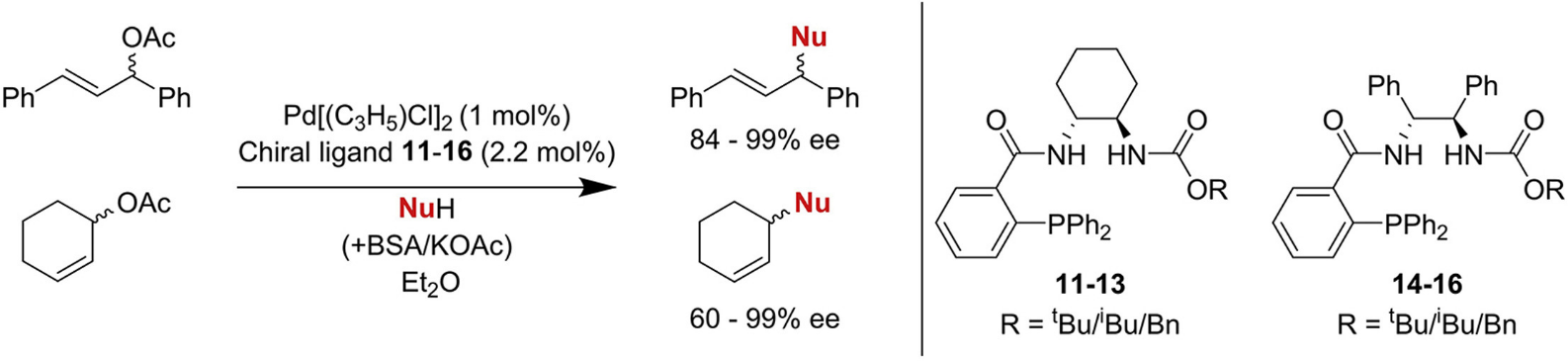
Carbamate-based P,O-ligands for asymmetric allylic alkylations
Tetrahedron (2020) 76, 131246
Herein we report the design and successful catalytic application of modified Trost-ligands in asymmetric allylic alkylation (AAA) reactions. A small set of carbamate-monophosphine P,O-ligands has been prepared in a straightforward two-step synthetic procedure. After optimization of the reaction conditions, high catalytic activities and excellent enantioselectivity up to >99% have been attained.
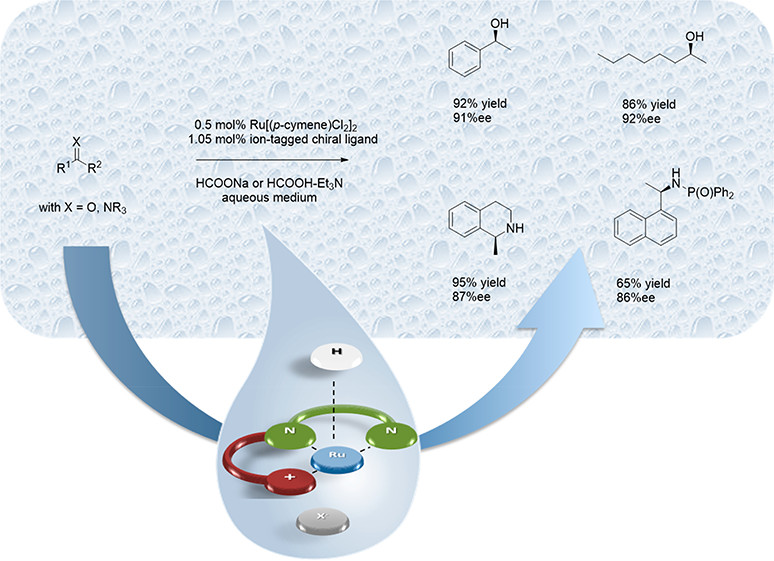
Ion-Tagged Chiral Ligands for Asymmetric Transfer Hydrogenations in Aqueous Medium
ACS Sustainable Chem. Eng. (2019) 7, 3414
We report the design and synthesis of novel ion-tagged chiral ligands for asymmetric transfer hydrogenation (ATH) in aqueous medium. Based on (R,R)-1,2-diphenylethylene diamine (DPEN) as structural motif, a straightforward three-step protocol was developed that gave access to novel chiral ligands with carbamate-substructure and pyridinium headgroup. The careful optimization of steric and electronic properties in combination with the adaption of solubility via choice of the anion gave a set of chiral and water-soluble ligands for use in ruthenium-catalyzed asymmetric transfer hydrogenations in aqueous medium. Eventually, a pool of aliphatic and aromatic ketones as well as two imine substrates were reduced with excellent isolated yields up to 95% and enantioselectivities >90% ee under environmentally benign conditions in the absence of additional surfactants.
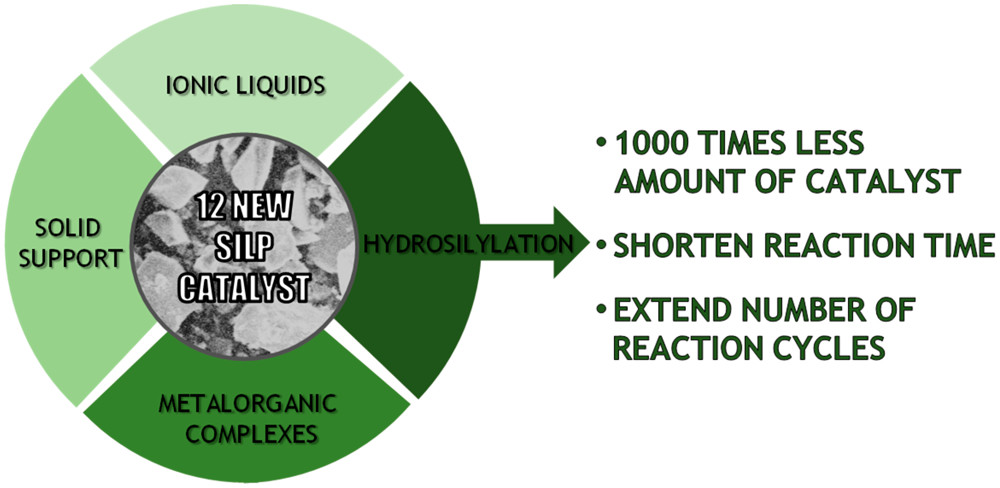
Highly Effective Supported Ionic Liquid-Phase (SILP) Catalysts: Characterization and Application to the Hydrosilylation Reaction
ACS Sustainable Chem. Eng. (2019) 7, 4699
Organosilicon compounds, because of their unique properties, are widely used in a variety of organic processes, and thus the constant improvement of current methods is still needed. We present slurry-phase hydrosilylation reactions using novel supported ionic liquid-phase (SILP) catalysts containing rhodium complexes immobilized in four phosphonium ionic liquids (ILs) on silica support. The obtained new SILP catalysts were analyzed by infrared technique, low-temperature nitrogen physisorption at 77 K, and scanning electronic microscopy with energy-dispersive X-ray spectroscopy to provide structural information on these materials. Moreover, the catalytic activity in hydrosilylation reactions was evaluated and compared with the catalytic activity of rhodium catalysts dissolved in the same ILs when using a biphasic reaction system (IL/catalyst as one phase and mixture of substrates as a second phase). The rhodium-based SILP catalysts proved to be much more efficient than when used in a biphasic system composed of a similar catalyst and reactants. Furthermore, as a result of the presented study, we have identified a highly active SILP catalyst ([{Rh(μ-OSiMe3)(cod)}2]/[P66614][NTf2] supported on silica) that allowed us to decrease the amount of catalyst used in the reaction by 1000 times in comparison with the amount of catalyst required while performing reaction using the biphasic catalytic system. The proposed method of utilization of SILP materials can become a significant step in reducing expensive organometallic catalyst consumption in organic chemistry and, when applied more broadly, lead to significant cost savings, eventually making the production of many organic molecules more sustainable.
FI-ICP-OES determination of Pb in drinking water after pre-concentration using magnetic nanoparticles coated with ionic liquid
Microchem. J. (2019) 146, 339
Accurate measurement of trace metals in environmental, biological and medical samples often requires the application of enrichment and/or isolation procedures. Among the many pre-concentration and/or matrix separation techniques available, solid phase extraction procedures (SPE) are most widely employed. In the last years, surface functionalized magnetic-nanoparticles have been introduced, which offer distinct improvements compared to conventional column-based SPE approaches. In the present work, a Flow-Injection (FI) procedure for on-line separation of magnetic nanoparticles from aqueous sample solutions with subsequent ICP-OES analysis is proposed. Silica-coated magnetic cobalt nanoparticles covered with an outer layer of ionic liquid were used as sorbent material. After analyte extraction, the particle suspension was directed through an electro-magnetic trap, thereby the analyte loaded particles were separated from the sample solution. For analysis, the electro-magnet was switched off and the particles were transferred with the carrier solution to the ICP-OES used for element-specific online analysis. Compared to batch-wise methods, the FI-approach offers an additional enrichment step and requires less manual sample manipulation. Applicability of the proposed FI-ICP-OES procedure was demonstrated by the analysis of Pb in the reference material ERM-CA022a and tap-water samples collected from different districts in Vienna, Austria.
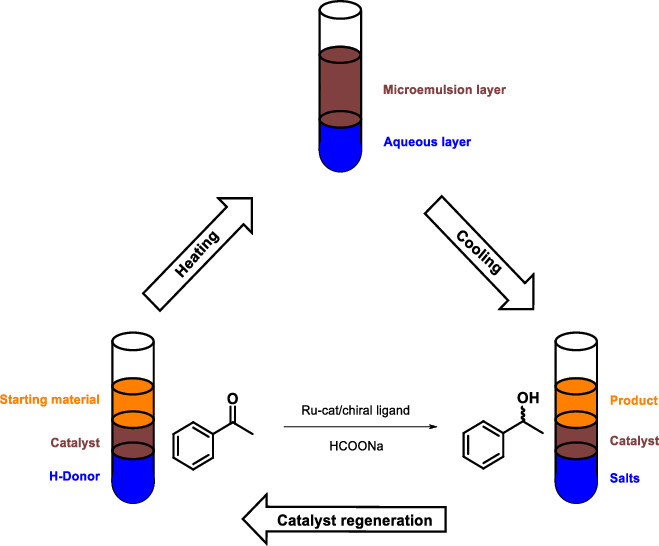
Asymmetric Transfer Hydrogenation in Thermomorphic Microemulsions Based on Ionic Liquids
Org. Process Res. Dev. (2019) 23, 1841
A thermomorphic ionic-liquid-based microemulsion system was successfully applied for the Ru-catalyzed asymmetric transfer hydrogenation of ketones. On the basis of the temperature-dependent multiphase behavior of the targeted microemulsion, simple product separation as well as catalyst recycling could be realized. The use of water-soluble ligands improved the immobilization of the catalyst in the microemulsion phase and significantly decreased the catalyst leaching into the organic layer upon extraction of the product. Eventually, the optimized microemulsion system could be applied to a wide range of aromatic ketones that were reduced with good isolated yields (up to 98%) and enantioselectivities (up to 97%), while aliphatic ketones were less successful.
Influence of the Ionic Liquid on the Activity of a Supported Ionic Liquid Phase FeII Pincer Catalyst for the Hydrogenation of Aldehydes
Zita Csendes, Julian Brünig, Nevzat Yigit, Günther Rupprechter, Katharina Bica-Schröder, Helmuth Hoffmann*, Karl Kirchner*
Eur. J. Inorg. Chem. (2019) 2019, 3503
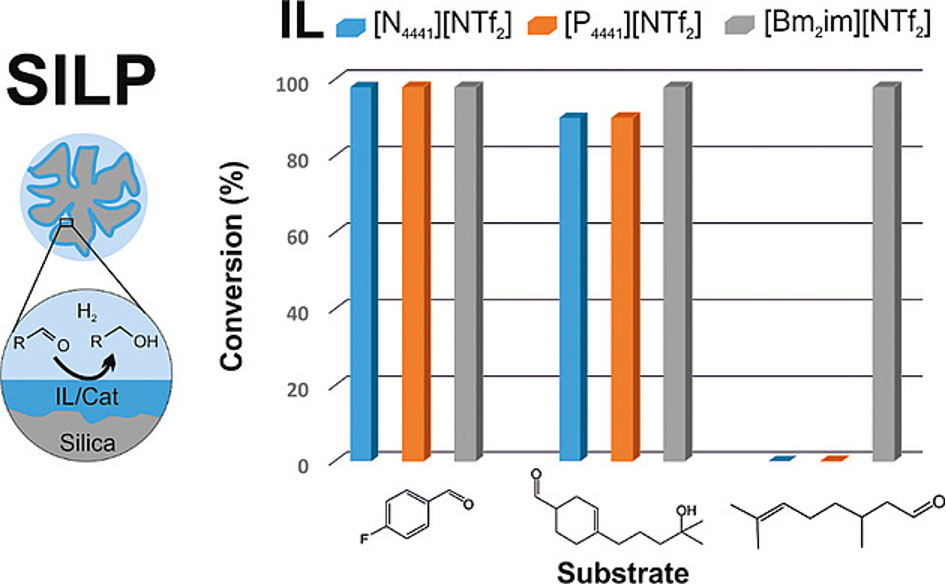
The catalytic hydrogenation of different aldehydes to the corresponding alcohols was investigated using an FeII hydride pincer complex as catalyst in the supported ionic liquid phase (SILP) reaction mode. Two different ionic liquids of the type [X4441][NTf2] with X=N or P were applied with mesoporous silica gel as support, which was coated first with a chemisorbed monolayer of the corresponding modified IL to remove acidic surface OH-groups and to prevent IL leaching. Quantitative conversion with turn-over frequencies in the order of 1000 h–1 were obtained for various aromatic and heteroaromatic aldehydes and highly selective aldehyde reduction was observed also for substrates containing reducible C=C bonds. Aldehydes with longer aliphatic chains or cycloalkyl substituents, however, showed no conversion here, in contrast to a previous study with an imidazolium-based ionic liquid. These differences were ascribed primarily to differences in substrate/ionic liquid interactions. Whereas [N4441][NTf2] and [P4441][NTf2] gave essentially identical results for different substrates in single-batch reactions, prolonged use of the catalyst in repeated reaction cycles lead to a quick drop-off in catalyst activity in [P4441][NTf2], but a continuous, quantitative conversion in [N4441][NTf2].
Simple lysis of bacterial cells for DNA-based diagnostics using hydrophilic ionic liquids
Sci. Rep. (2019) 9, 13994
Carbon-based SILP catalysis for the selective hydrogenation of aldehydes using a well-defined Fe(ii) PNP complex
Catal. Sci. Technol. (2018) 8, 4812
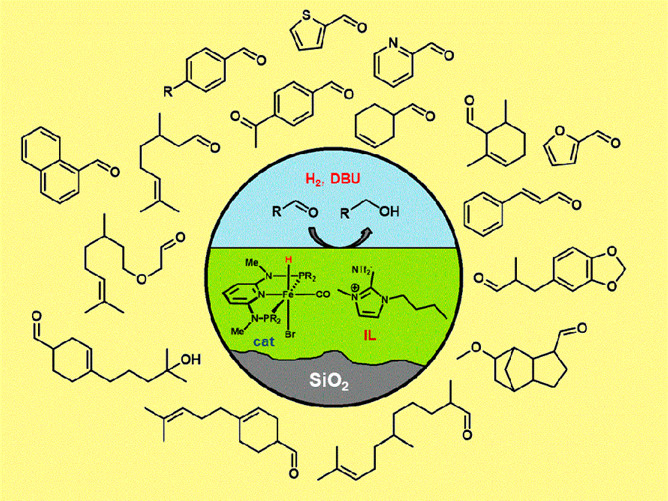
In this work, the supported ionic liquid phase (SILP) method was applied for the immobilization of a newly developed, well-defined hydride Fe(II) PNP pincer complex dissolved in ionic liquid (IL) onto polymer-based spherical activated carbon. This novel SILP catalyst was structurally characterized by electron microscopy, N2 adsorption–desorption, FTIR, and XPS measurements and was used for the hydrogenation of aldehydes to alcohols. For an optimized pore filling degree, this system showed excellent activity in the chemoselective hydrogenation of different aldehydes and proved to be reusable in at least seven consecutive reaction cycles without any loss in activity. Significantly lower reaction rates were observed, however, compared to a recent study of the same catalyst supported on silica, which was ascribed to the different pore sizes of these two support materials.
Chemoselective Supported Ionic-Liquid-Phase (SILP) Aldehyde Hydrogenation Catalyzed by an Fe(II) PNP Pincer Complex
ACS Catal. (2018) 8, 1048
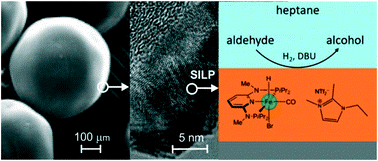
A base-tolerant supported ionic-liquid-phase (SILP) system containing a well-defined hydride Fe(II) PNP pincer complex has been prepared, structurally characterized, and used as catalyst in the hydrogenation of aldehydes to alcohols. The new SILP catalyst, with the optimum pore filling, was highly active exhibiting TONs and TOFs of up to 1000 and 4000 h–1, respectively, under mild conditions (25 °C, 10–50 bar H2 pressure) without significant leaching of both the complex and the IL.
Electrochemical properties of halogenated benzylidenehydrazino-pyrazoles in various imidazolium-based ionic liquids
Monatsh. Chem. (2018) 149, 823
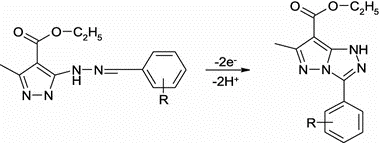
The present paper deals with the anodic oxidation of various benzylidenehydrazino-pyrazoles in several room-temperature ionic liquids containing the imidazolium scaffold towards a more environmentally friendly route in obtaining the corresponding pyrazolo-triazoles. We have studied the voltammetric response of the latter substrates on a platinum disc electrode by varying the nature of the imidazolium cation to find the most suitable reaction medium. By changing the substituents attached to the para-position of the phenyl ring within the substrates, we intended to better understand the nature of the formed intermediate and to elucidate the mechanism of their electrode reaction.
Surface-Active Ionic Liquids in Catalytic Water Splitting
Aust. J. Chem. (2018) 72, 34
We report the application of surface-active ionic liquids as ligands and optional reaction media in iridium-catalyzed water oxidations. Three novel catalysts with N,N-dialkylimidazolidin-2-ylidene ligands based on amphiphilic imidazolium ionic liquids were synthesized and characterized. Excellent turn-over frequencies of up to 0.92 s−1 were obtained in catalytic water splitting, and activity was maintained for five consecutive catalytic cycles, with an overall turn-over number of 8967. The addition of external surface-active ionic liquid showed unexpected behaviour, because strongly enhanced initial reaction rates were observed.
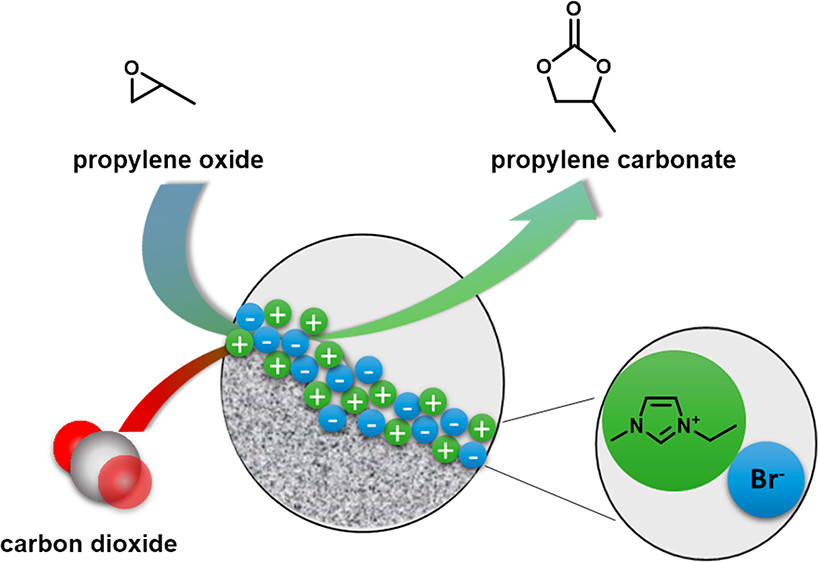
Continuous Conversion of Carbon Dioxide to Propylene Carbonate with Supported Ionic Liquids
ACS Sustainable Chem. Eng. (2018) 6, 13131
We present the use of silica-supported ionic liquids as catalysts for the continuous production of propylene carbonate from propylene oxide using supercritical carbon dioxide as solvent and reagent. Considerable differences in the catalytic activity of ionic liquids in homogeneous catalysis in batch mode and in continuous-flow using supported species processes were found, suggesting that a synergistic effect between ionic liquid and silica support material takes place. While supported ionic liquids prepared via physisorption of [C2mim]Br showed the highest catalytic activity, studies on long-term stability showed a rapid loss in yield due to the formation of undesired polypropylene carbonate that agglomerated in the ionic liquid layer. Improved long-term stability was found for ionic liquids covalently bound to the silica support materials, suggesting that a compromise between activity and stability is the best solution for the continuous production of propylene carbonate.
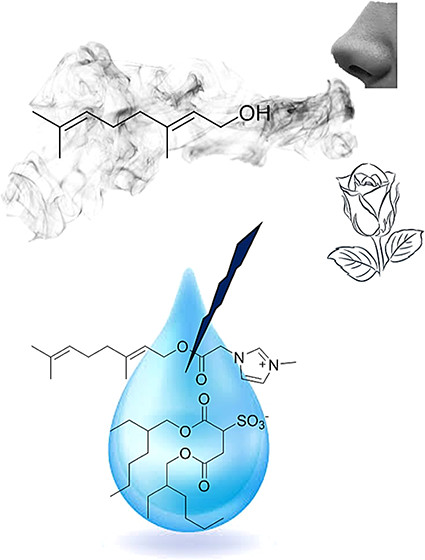
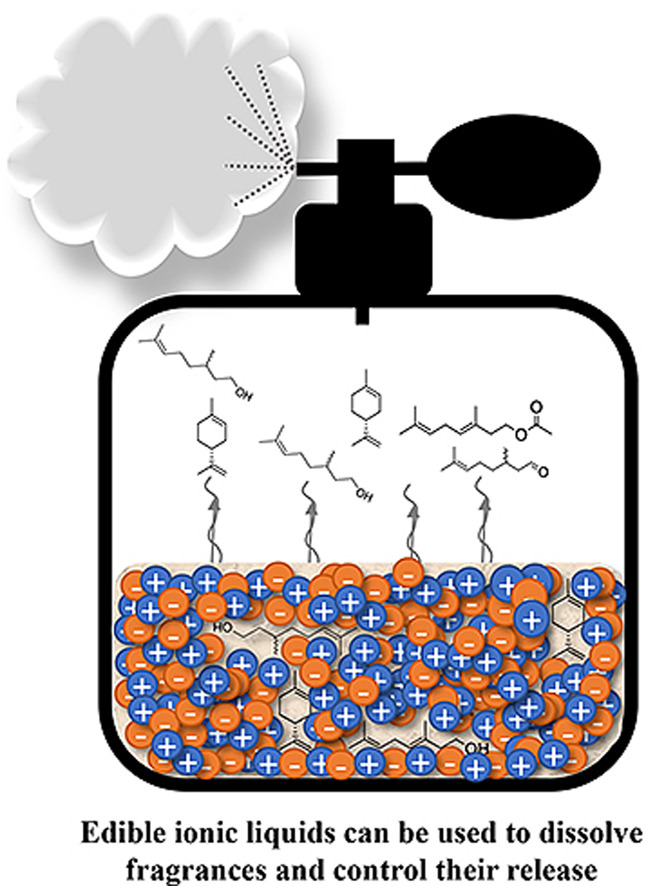
Ionic Liquids as Fragrance Precursors: Smart Delivery Systems for Volatile Compounds
Ind. Eng. Chem. Res. (2018) 57, 16069
The low volatility of many ionic liquids (ILs) can be used to design a specific delivery system with a triggered release mechanism for volatile fragrances. We present two different strategies for IL-supported profragrance compounds based on common fragrance alcohols. When the fragrance was attached to the cation, its release was triggered by heat, while the fragrance attached to the anion was released under basic conditions. Selection of the counterion allowed not only control of the physical properties of the resulting compound but also addition of a second functionality. Our design strategy shows that the choice of the IL components can result in safe, biocompatible ionic profragrances suitable for applications such as topical nutraceutical or cosmetic formulations.
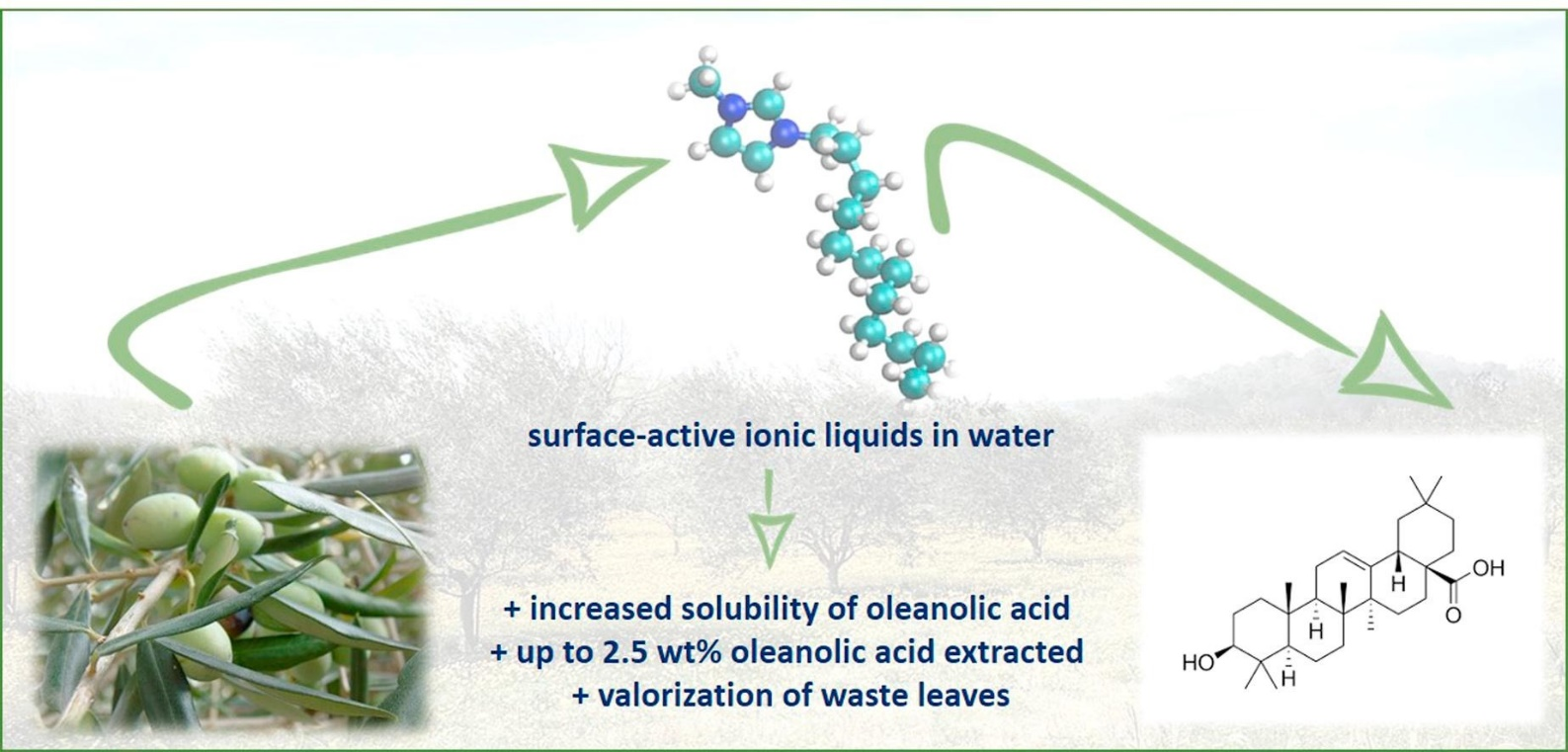
Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids
Sep. Purif. Technol. (2018) 204, 30
The global olive oil industry annually generates approximately 750,000–1,500,000 tons of Olea europaea leaves as waste that are typically burned for energy production. Yet, this agricultural by-product is a rich source of oleanolic acid, a high value triterpenic acid with outstanding pharmaceutical and nutraceutical activities. The present study focuses on the extraction of oleanolic acid from dried O. europaea leaves using aqueous solutions of surface-active ionic liquids as alternative solvents. A number of imidazolium-based ionic liquids with variable chain length, different anions and optional side-chain functionalization was synthesized and employed in the extraction of oleanolic acid. Ionic liquids with long alkyl chains remarkably enhance the solubility of oleanolic acid in water, thus being able to compete with the solubilities afforded by molecular organic solvents, such as chloroform. Consequently, they are suitable alternatives for the solid-liquid extraction of triterpenic acids from natural matrices and provide improved extraction yields of up to 2.5 wt% oleanolic acid extracted from olive tree leaves.
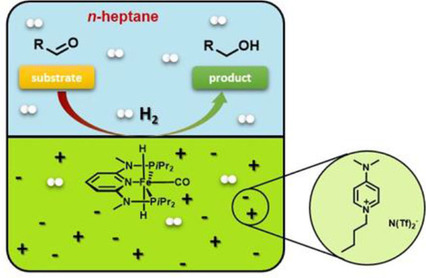
Selective Hydrogenation of Aldehydes Using a Well-Defined Fe(II) PNP Pincer Complex in Biphasic Medium
ChemCatChem (2018) 10, 4386
A biphasic process for the hydrogenation of aldehydes was developed using a well-defined iron (II) PNP pincer complex as model system to investigate the performance of various ionic liquids. A number of suitable hydrophobic ionic liquids based on the N(Tf)2− anion were identified, allowing to immobilize the iron (II) catalyst in the ionic liquid layer and to facilitate the separation of the desired alcohols. Further studies showed that targeted Brønsted basic ionic liquids can eliminate the need of an external base to activate the catalyst.
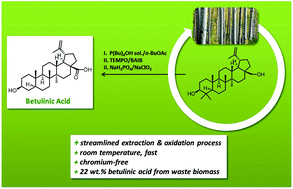
Toward a benign strategy for the manufacturing of betulinic acid
Green Chem. (2017) 19, 1014
We report a novel and efficient strategy for the preparation of the high-value triterpenoid betulinic acid based on extraction and streamlined oxidation of betulin from the industrial by-product birch bark. The initial extraction of betulin relies on a biphasic system and allowed extracting betulin in short times at room temperature. The crude extract could be directly oxidized, thereby providing a chromium-free, time- and energy saving strategy for the manufacturing of betulinic acid in high yield.
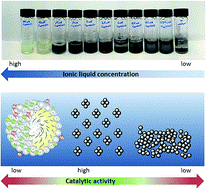
Surface-active ionic liquids for palladium-catalysed cross coupling in water: Effect of ionic liquid concentration on the catalytically active species
RSC Adv. (2017) 7, 41144
We report the design and synthesis of surface-active ionic liquids for application in palladium-catalyzed cross coupling reactions. A series of dodecylimidazolium-based ionic liquids were applied as additives in the Heck reaction of ethyl acrylate and iodobenzene, and high yields of >90% could be obtained in water without the addition of further ligands. Our results indicate that the ionic liquid concentration in water is the key factor affecting the formation of the catalytically active species and hence the yield. Moreover, imidazolium-based ionic liquids that are able to form a carbene species differ significantly from conventional cationic surfactants, as a concentration dependent formation of the N-heterocyclic carbene complex was observed.
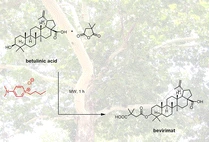
Design and synthesis of basic ionic liquids for the esterification of triterpenic acids
Monatsh. Chem. (2017) 148, 139
We present the design and synthesis of Brønsted-basic ionic liquids and investigate their application in the microwave-assisted esterification of betulinic acid, aiming towards a benign and pyridine-free manufacturing process of the anti-HIV drug, bevirimat.
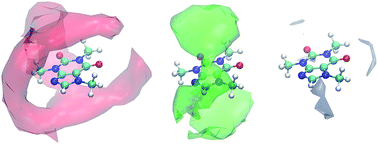
Computational analysis of the solvation of coffee ingredients in aqueous ionic liquid mixtures
RSC Adv. (2017) 6, 3495
In this paper, we investigate the solvation of coffee ingredients including caffeine, gallic acid as representative for phenolic compounds and quercetin as representative for flavonoids in aqueous mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate [C2mim][OAc] at various concentrations. Due to the anisotropy of the solutes we show that classical Kirkwood–Buff theory is not appropriate to study solvation effects with increasing ionic liquid content. However, excess coordination numbers as well as the mean residence time of solvent molecules at the surface of the solutes can be determined by Voronoi tessellation. Since the volume of the hydration shells is also available by this method, solvation free energies will be discussed as a function of the ionic liquid concentration to yield a physical meaningful picture of solvation for the anisotropic solutes. Hydrogen bonding capabilities of the solutes and their relevance for experimental extraction yields from spent coffee grounds are also discussed.
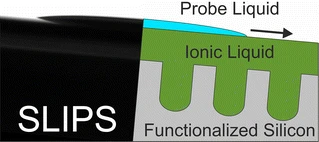
Fluorine-free, liquid-repellent surfaces made from ionic liquid-infused nanostructured silicon
Monatsh. Chem. (2017) 148, 167
Liquid-repellent surfaces based on slippery liquid-infused porous substrates (SLIPS) were prepared from porous, nanostructured silicon surfaces with different surface functionalization, infused with the polar ionic liquid 1-ethyl-3-methylimidazolium methylsulfate ([C2mim]MeSO4). Contrary to nonpolar SLIPS based on perfluorinated substrates and infusion liquids, [C2mim]MeSO4 forms stable SLIPS with high energy surfaces like native silicon (Si–SiO2) or ionic liquid-functionalized silicon (Si-[C3mim]Cl), whose liquid-repellent properties against low surface tension liquids (toluene, cyclohexane) were demonstrated by very low sliding angles (α < 3°) and low contact angle hysteresis (Δθ < 10°). These polar, ionic liquid-based SLIPS present a promising, environmentally benign extension of liquid-infused substrates to natural, high-energy oxide surfaces.
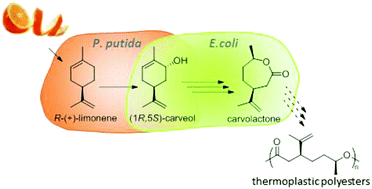
From waste to value – direct utilization of limonene from orange peel in a biocatalytic cascade reaction towards chiral carvolactone
Green Chem. (2017) 19, 367
In this proof of concept study we demonstrate direct utilization of limonene containing waste product orange peel as starting material for a biocatalytic cascade reaction. The product of this cascade is chiral carvolactone, a promising building block for thermoplastic polymers. Four different concepts were applied to augment limonene availability based on either water extraction solely, addition of extraction enhancers or biomass dissolution.
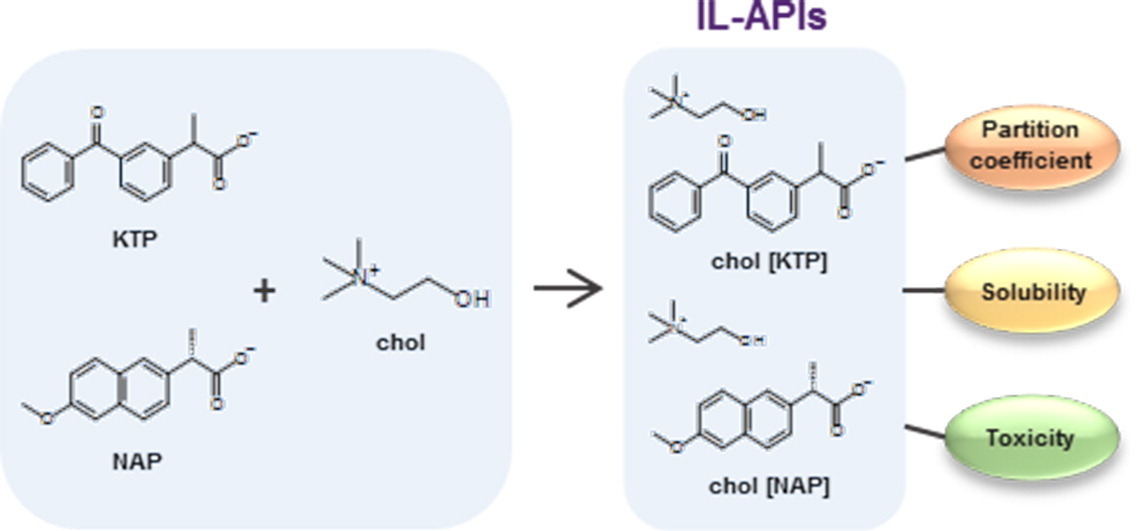
Anti-inflammatory choline based ionic liquids: Insights into their lipophilicity, solubility and toxicity parameters
Ana M.O. Azevedo, Susana P.F. Costa, Ana F.V. Dias, Alexandre H.O. Marques, Paula C.A.G. Pinto, Katharina Bica, Anna K. Ressmann, Marieta L.C. Passos, André R.T.S. Araújo, Salette Reis, M. Lúcia M.F.S. Saraiva*
J. Mol. Liq. (2017) 232, 20
The impact on in vivo efficacy and safety of two novel ionic liquids based on the association of choline with non-steroidal anti-inflammatory drugs, ketoprofen and naproxen forming IL-APIs, was evaluated. Their lipophilicity, solubility and toxicity were assessed aiming the illustration of the pharmaceutical profile and potential toxic impact.
Partition coefficient was determined using micelles of hexadecylphosphocholine and UV–Vis derivative spectroscopy. Additionally, solubility in phosphate buffer pH 7.4 was measured using a modified shake flask method and UV–Vis spectroscopy as detection technique. Ultimately, toxicity was considered resorting to a fully automated cytochrome c oxidase assay based on microfluidics. The obtained results demonstrated that the IL-APIs’ drug format has the ability to interact with biological membranes and also improves solubility up to 58 times. Moreover, it was evidenced that, although being a nutrient, choline influences the IL-APIs’ toxicity. The studied anti-inflammatory IL-APIs exhibited promising properties regarding their incorporation in pharmaceutical formulations.
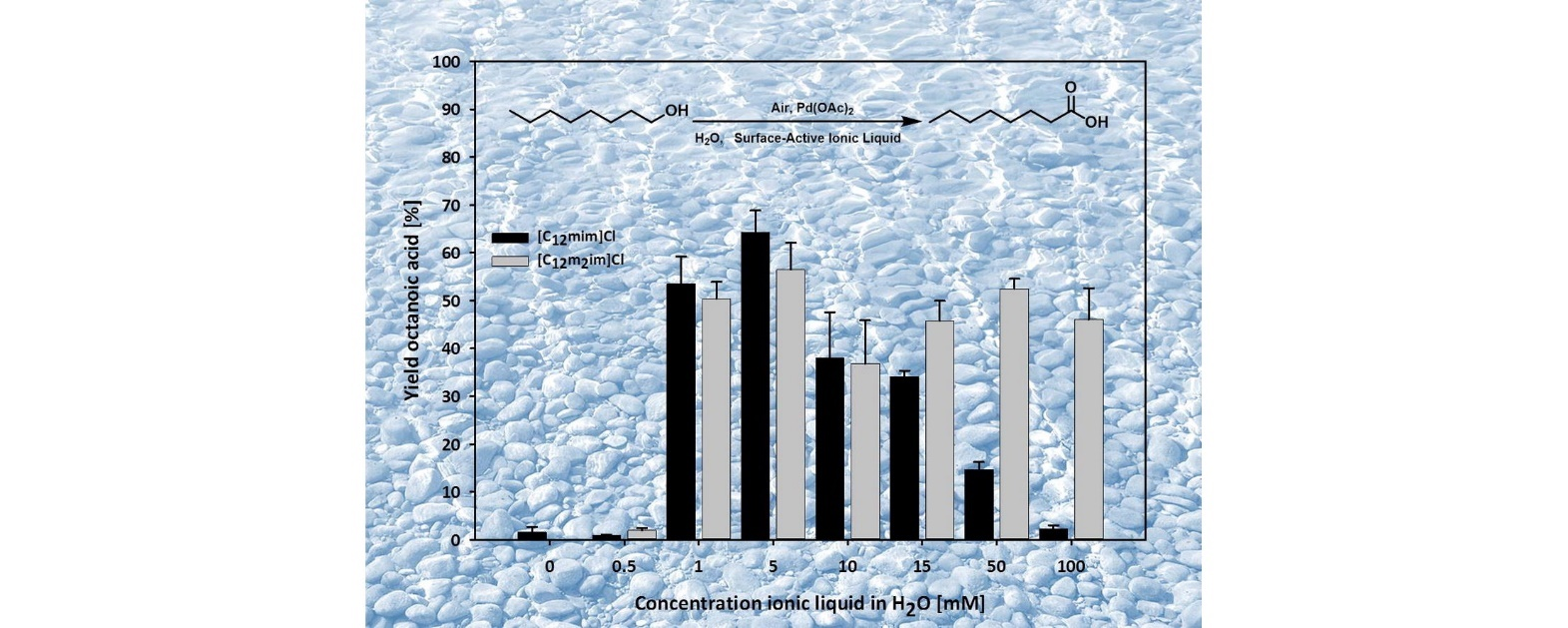
Surface-active ionic liquids in catalysis: Impact of structure and concentration on the aerobic oxidation of octanol in water
J. Colloid Interface Sci. (2017) 492, 136
We present design and synthesis of surface-active ionic liquids for the application in micellar catalysis. A series of 1-methyl-3-dodecylimidazolium based ionic liquids with variable core structures including dicationic and zwitterionic ones was synthesized and characterized. These surface-active ionic liquids where applied in the aerobic oxidation of aliphatic alcohols to carbonyl compounds. A strong dependence on the ionic liquid concentration and structure was identified, which is in accordance with the concepts of micellar catalysis. Optimum conditions for the oxidation of 1-octanol could be identified, and the use of surface-active ionic liquids strongly improved the reaction performance compared to pure water. Under optimized conditions, it was possible to isolate up to 75% of octanoic acid using only small amounts of surface-active ionic liquid in a 0.05 mM solution in water without further ligands.
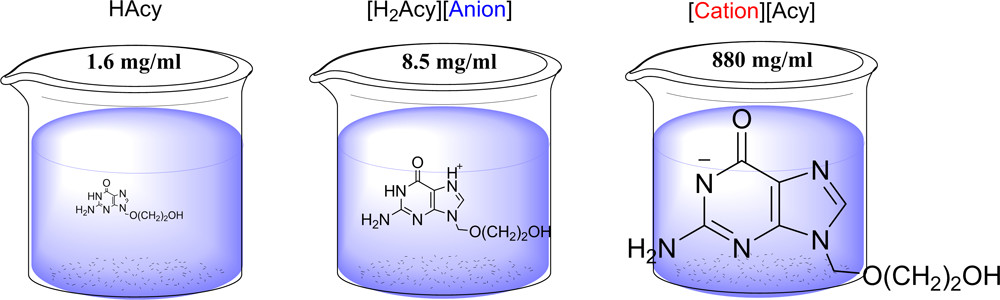
Acyclovir as an Ionic Liquid Cation or Anion Can Improve Aqueous Solubility
ACS Omega (2017) 2, 3483
Six ionic liquid (IL)-forming ions (choline, tetrabutylphosphonium, tetrabutylammonium, and trimethylhexadecylammonium cations, and chloride and docusate anions) were paired with acyclovir as the counterion to form four low melting solid salts and two waxes; five of these compounds could be classified as ILs. All of the newly synthesized acyclovir ILs exhibited increased aqueous solubilities by at least 2 orders of magnitude when compared to that of neutral acyclovir. For three of the prepared compounds, the solubilities in simulated body fluids (phosphate-buffered saline, simulated gastric, and simulated intestinal fluids) were also greatly enhanced when compared to that of neutral acyclovir. Acyclovir in its anionic form was more water- or buffer-soluble than acyclovir in its cationic form, though this might be the effect of the particular ions, indicating that the solubilities can be finely tuned by proper choice of the cationic or anionic form of acyclovir and the counterion paired with it.
Hydration and Counterion Binding of [C12MIM] Micelles
Langmuir (2017) 33, 9844
Surface-active ionic liquids based on imidazolium cations are promising targets for micellar catalysis in aqueous solution, yielding enhanced rate constants compared to surfactants based on n-alkyltrimethylammonium cations and exhibiting a pronounced counterion dependence (Bica Chem. Commun. 2012, 48, 5013−5015; Cognigni Phys. Chem. Chem. Phys. 2016, 18, 13375–13384). Probably most relevant to these effects is the interplay between headgroup hydration and counterion binding. To obtain more detailed information on these effects, aqueous solutions of 1-dodecyl-3-methylimidazolium ([C12MIM]) bromide, iodide, and triflate (TfO–) were investigated at 45 °C using broadband dielectric spectroscopy, viscosity measurements, and small-angle X-ray scattering experiments. Effective hydration numbers were determined, and information on the locations and mobilities of the condensed counterions, X–, was derived. It was found that [C12MIM] halide micelles were less hydrated than the corresponding n-dodecyltrimethylammonium ([C12TA]X) aggregates. Together with their somewhat weaker counterion condensation, this difference probably explains their higher catalytic activity. Whereas [C12MIM]Br micelles remained roughly spherical in the studied concentration range, rodlike aggregates were formed at high concentrations of the iodide and, in particular, the triflate surfactants. It appears that the much lower mobility of condensed TfO– counterions is the reason for the very low catalytic activity of [C12MIM]TfO micelles.
Ionic liquids for consumer products: Dissolution, characterization, and controlled release of fragrance compositions
Fluid Phase Equilib. (2017) 450, 51
Ionic liquids composed entirely of pharmaceutically-approved ions were used for the dissolution and storage of volatile fragrance components with improved thermal stability and prolonged release. The presence of the cation of the ionic liquid in the head space was also observed, highlighting the need for careful selection of the components of the ionic liquid for consumer products.
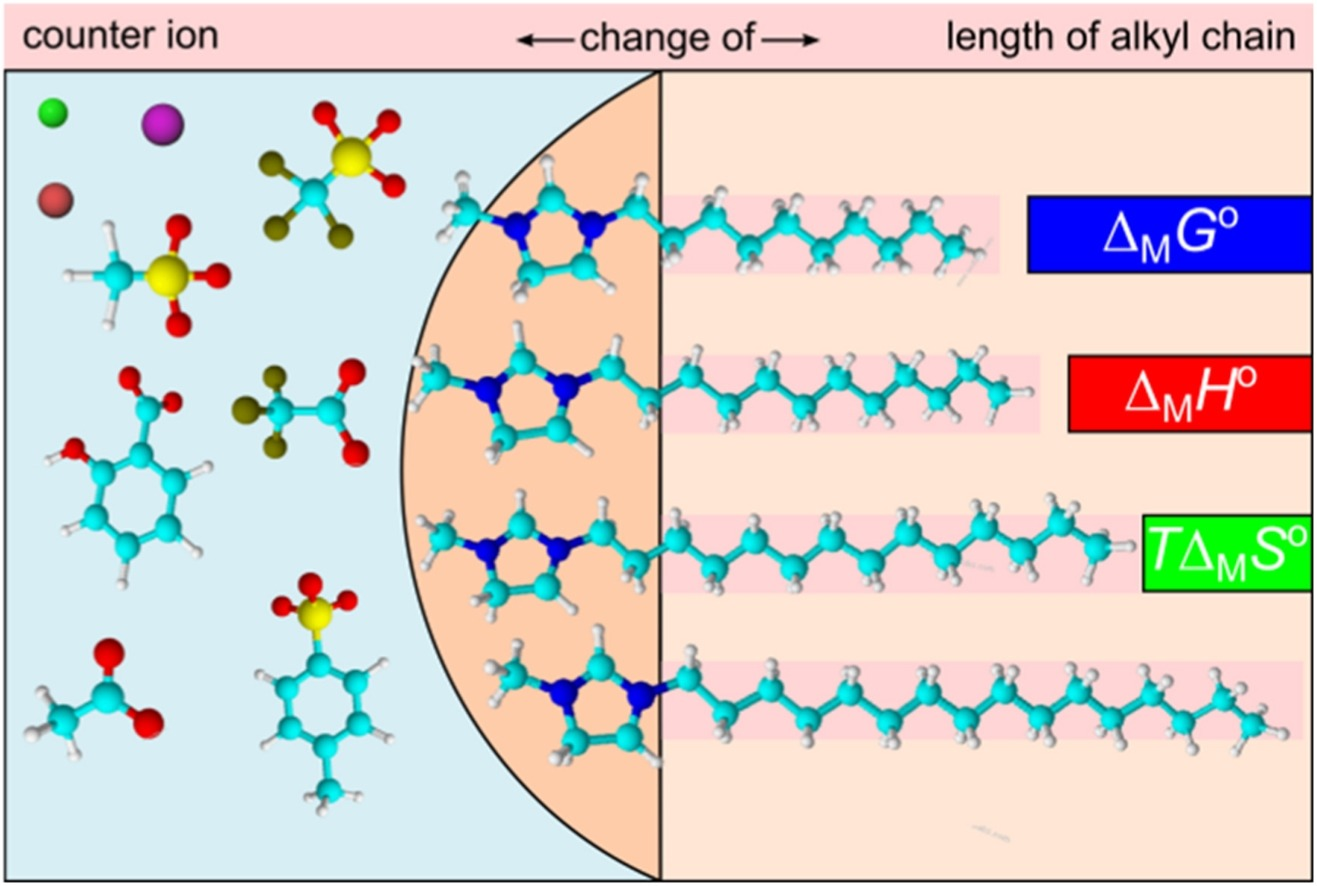
Thermodynamic study for micellization of imidazolium based surface active ionic liquids in water: effect of alkyl chain length and anions
Colloids Surf., A (2017) 532, 609
A systematic investigation of influence of the length of the side alkyl chain and the counter-ions on the thermodynamics of the micellization process at imidazolium based surface active ionic liquids (SAILs) in aqueous solutions was carried out by isothermal titration calorimetry (ITC) in a broad temperature range.
The effect of alkyl chain length on the micellization process in water has been investigated on 1-decyl-3-methylimidazolium ([C10mim]Cl), 1-dodecyl-3-methylimidazolium ([C12mim]Cl), 1- tetradecyl-3-methylimidazolium ([C14mim]Cl) and 1-hexadecyl-3- methylimidazolium ([C16mim]Cl) chlorides, whereas the influence of counter-ion was studied at the micellization of 1-dodecyl-3-methylimidazolium chloride ([C12mim]Cl), bromide ([C12mim]Br), iodide ([C12mim]I), acetate ([C12mim]OAc), methanesulfonate ([C12mim]OMs), toluen sulfonate ([C12mim]OTs), trifluromethane sulfonate ([C12mim]OTf), trifluoro acetate ([C12mim]TFA) and salicylate ([C12mim]Sal) in water.
From ITC experimental data, the corresponding standard thermodynamic parameters of micellization (enthalpy, ΔMH0; Gibbs free energy, ΔMG0; entropy, ΔMS0; heat capacity change, ΔMcp0) were estimated by help of the mass-action model. It was found that SAILs behave mainly like common cationic surfactants, where cmc values are decreasing with the length of the side alkyl chain and exhibit U-shape dependence on temperature. Remarkable influence of counter-ions on cmc and all thermodynamic functions was observed, however the entropy-enthalpy compensation turned out as a matter of fact, arising from the relationship ΔG = ΔH − TΔS. ΔMcp0 values were further discussed in terms of the removal of solvent accessible surface areas of SAILs and counter-ion from the contact with water after micellization.
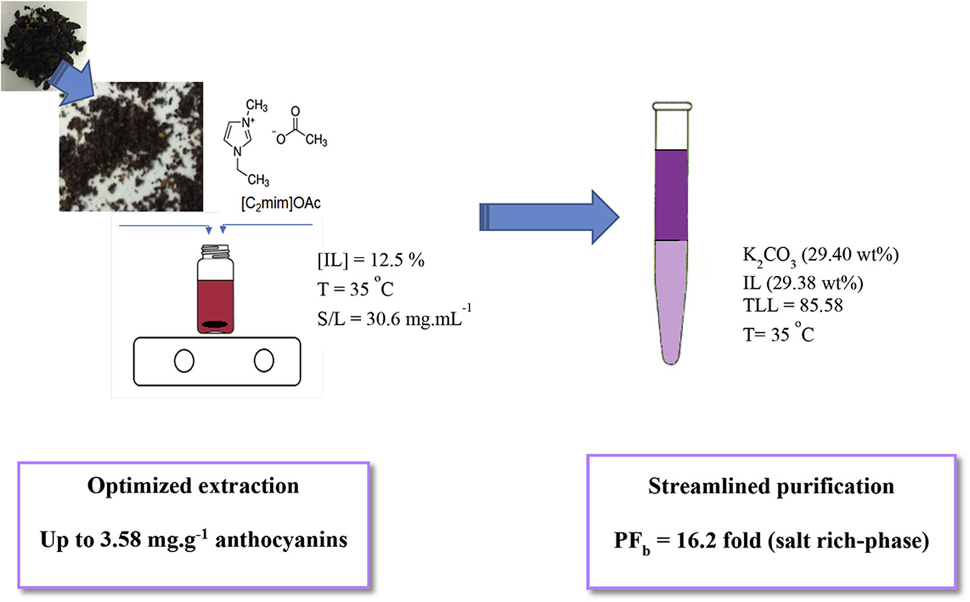
Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions
Fluid Phase Equilib. (2017) 451, 68
The global wine industry generates approximately 8.5 million tons of waste annually, which contains several biomolecules of high value. The present study focuses on the extraction of anthocyanins from grape pomace via solid/liquid extraction with aqueous solutions of the ionic liquid 1-ethyl-3-methylimidzaolium acetate [(C2mim]OAc) and sequential purification in aqueous two-phase systems (ATPS) with additional salts. The optimum conditions for solid/liquid extraction were obtained using response surface methodology and yielded up to 3.58 mg g−1 anthocyanins. During ATPS formation, the highest purification factor (PF = 16.2 fold) was obtained in the bottom and salt-rich phase using K2CO3 (29.41 wt%) and [C2mim]OAc (29.28 wt%) at 35 °C and atmospheric pressure. The partition process is exothermic, spontaneous and governed by enthalpy forces.
A comparison of two methods of recovering cobalt from a deep eutectic solvent: Implications for battery recycling
Franziska-Jane Albler, Katharina Bica, Mark R.StJ. Foreman*, Stellan Holgersson, Mikhail S. Tyumentsev
J. Clean. Prod. (2017) 167, 806
Fast and efficient extraction of DNA from meat and meat derived products using aqueous ionic liquid buffer systems
New J. Chem. (2015) 39, 4994
A short, simple and inexpensive process for the extraction of DNA from meat was developed investigating a set of 20 ionic liquids including imidazolium, choline and guanidinium derivatives in combination with aqueous buffer systems. The environmentally benign ionic liquid choline hexanoate–phosphate buffer mixture gave the most promising results and DNA was extracted within 20 minutes from different types of meats, such as beef, chicken, pork and horse in significantly higher yields compared to the pure phosphate buffer. The influence of the ionic liquid on the amplification process during the PCR was further investigated, showing an inhibitory effect with increasing chain length of the ionic liquid and with higher ionic liquid concentrations. Additionally, extracted DNA was stable for 20 days when stored at room temperature in aqueous ionic liquid–buffer mixtures.

Coordinating chiral ionic liquids: design, synthesis, and application in asymmetric transfer hydrogenation under aqueous conditions
Eur. J. Org. Chem. (2015) 2015, 2374
Hydrophilic coordinating chiral ionic liquids with an amino alcohol substructure were developed and efficiently applied to the asymmetric reduction of ketones. Their careful design and adaptability to the desired reaction conditions allow for these chiral ionic liquids to be used as the sole source of chirality in a ruthenium-catalyzed transfer hydrogenation reaction of aromatic ketones. When used in this reaction system, these chiral ionic liquids afforded excellent yields and high enantioselectivities.
Amino alcohol-derived chiral ionic liquids: structural investigations toward chiral recognition
Tetrahedron: Asymmetry (2015) 26, 1069
From tentative beginnings, chiral ionic liquids have emerged into functionalized and tailor-made materials that provide novel input in classical asymmetric synthesis and separations. However, despite this broad application range the prediction of any chiral ionic liquids’ performance is difficult. We present a systematic study toward the chiral recognition properties of novel ionic liquids with an amino alcohol sub-structure derived from the chiral pool precursors ephedrine, prolinol, and phenylalaninol. The influence of different ionic head groups and core structures on the diastereomeric interactions between racemic Mosher’s acid carboxylate and enantiopure chiral ionic liquids was systematically investigated to provide insight into their recognition properties prior to application of these chiral ionic liquids in various fields.
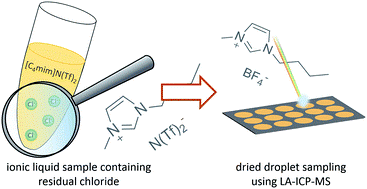
Determination of residual chloride content in ionic liquids using LA-ICP-MS
RSC Adv. (2016) 6, 90273
Nowadays, ionic liquids (ILs) are used in a wide range of applications. Their exceptional chemical and physical properties, sustainability of use and the possibility of easy recycling are attractive aspects promoting the acceptance of ILs also for commercial applications. While synthesis is in most cases simple and straightforward, purification of the reaction products might pose a number of problems. Due to the major influence of inorganic contaminations from the synthesis process, thorough monitoring of impurities is required. However, the unusual properties of ILs create some problems for conventional chemical analysis. In this work, a dried droplet approach with subsequent LA-ICP-MS sampling will be used for the analysis of chloride in ILs – a by-product from the synthesis procedure. Dried droplet application onto pre-cut filter paper disks allows the analysis of hydrophilic as well as hydrophobic ILs with calibration from dried aqueous standards for signal quantification. The approach is applied on two types of alkylimidazolium ILs, underlining the versatility of the sample preparation and measurement approach. Compared to commonly used analysis techniques for chloride in ILs, the presented approach is simple, fast, and does not require harmful reagents. Different internal standardization strategies were investigated during this study. With a reproducibility of below 2% relative standard deviation and limits of detection of 0.09 mg g−1 for chloride in ILs, the presented approach was showed to be fit for the purpose of routine analyses and reaction monitoring. If necessary, the approach can be extended to other analytes of interest in the field of the synthetic chemistry of ILs.
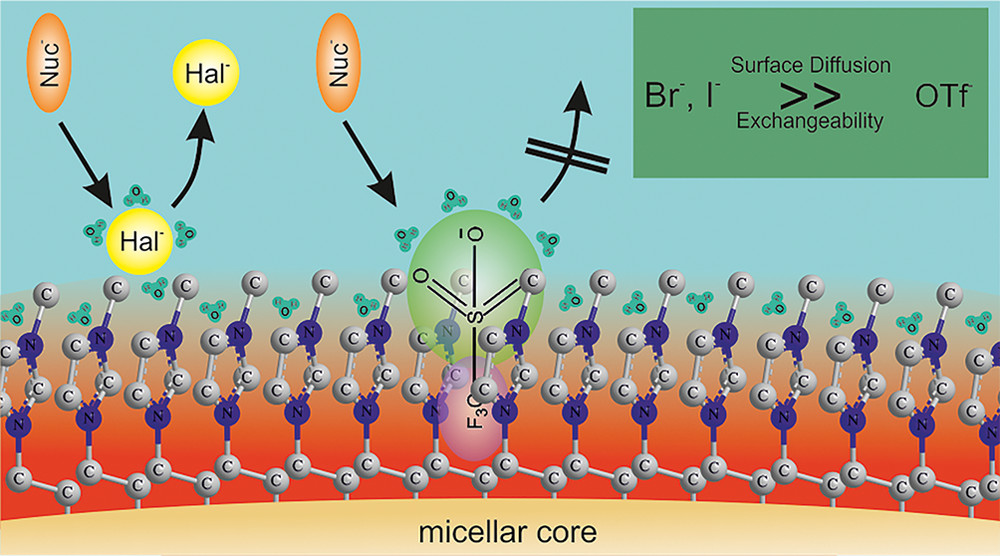
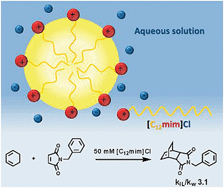
Surface-active ionic liquids in micellar catalysis: impact of anion selection on reaction rates in nucleophilic substitutions
Phys. Chem. Chem. Phys. (2016) 18, 13375
A series of surface-active ionic liquids based on the 1-dodecyl-3-methylimidazolium cation and different anions such as halides and alkylsulfates was synthesized. The aggregation behavior of these ionic liquids in water was characterized by surface tension, conductivity measurements and UV-Vis spectroscopy in order to determine the critical micelle concentration (CMC) and to provide aggregation parameters. The determination of surface activity and aggregation properties of amphiphilic ionic liquids was accompanied by SAXS studies on selected surface-active ionic liquids. The application of these surface-active ionic liquids with different anions was tested in nucleophilic substitution reactions for the degradation of organophosphorus compounds. Kinetic studies via UV-Vis spectrophotometry showed a strong acceleration of the reaction in the micellar system compared to pure water. In addition, an influence of the anion was observed, resulting in a correlation between the anion binding to the micelle and the reaction rate constants, indicating that the careful choice of the surface-active ionic liquid can considerably affect the outcome of reactions.
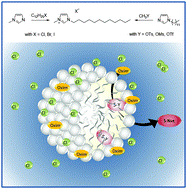
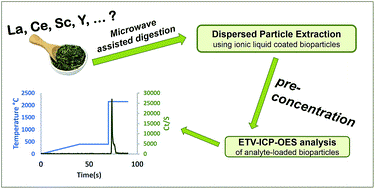
Bioparticles coated with an ionic liquid for the pre-concentration of rare earth elements from microwave-digested tea samples and the subsequent quantification by ETV-ICP-OES
Anal. Methods (2016) 8, 7808
An analytical procedure for straight-forward quantification of rare earth elements (REEs) in tea was developed. The method consists of three steps: first, dry tea powder is converted into an aqueous sample solution using a microwave-assisted digestion procedure. Then, the REEs are retained on newly designed sorbent particles and are thus effectively extracted from the sample digest. Then, the REE-loaded sorbent material is introduced into the furnace of an electro-thermal vaporization (ETV) unit. There, the core of the particles is pyrolyzed and removed in a first temperature step. Then, the REE analytes are swiftly evaporated at high temperatures, and subsequently analyzed by ICP-OES. The advantages of the method proposed here are as follows: extraction of REEs is accomplished via “dispersed particle extraction”; this avoids typical shortcomings of conventional solid-phase extraction. The analysis via ETV allows separation of the sorbent particles from the analytes in time. Thus, detrimental effects of plasma-loading are circumvented. The method was shown to provide satisfactory detection limits of typically 50 ng g−1 in the dry tea samples (3 s criterion). The method was validated by means of spike recovery experiments, and applied to the analysis of ten different tea samples from China, Japan and India, as sold in European markets.
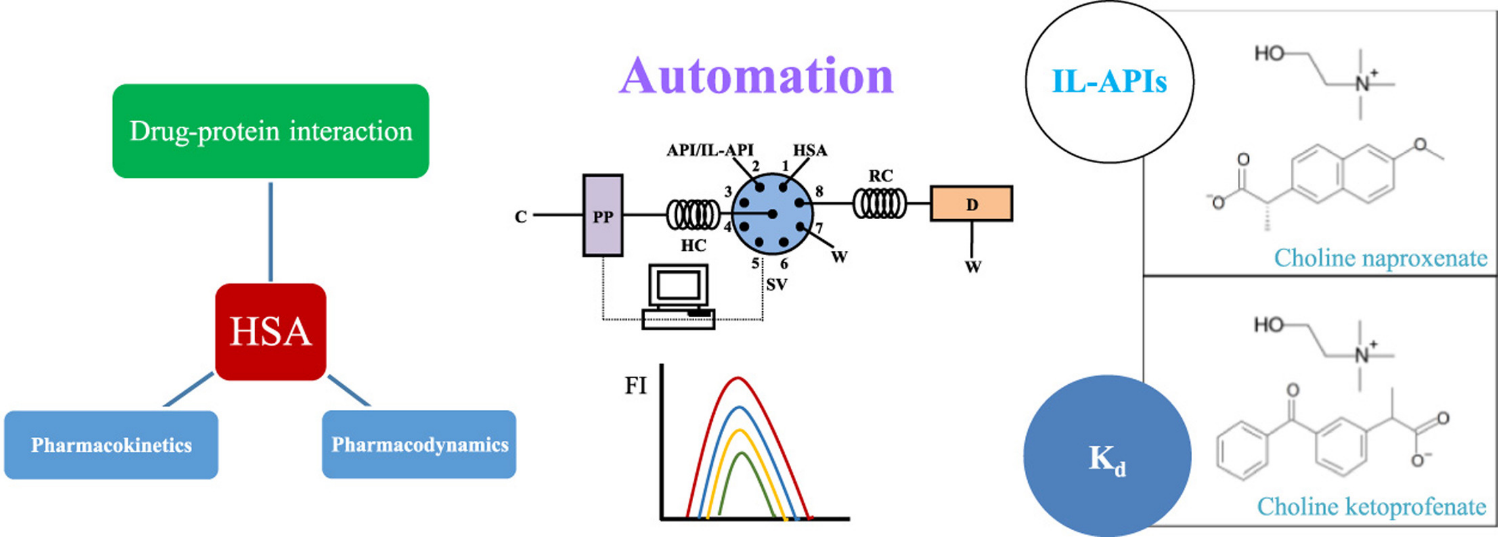
Automated evaluation of protein binding affinity of anti-inflammatory choline based ionic liquids
Talanta (2016) 150, 20
In this work, an automated system for the study of the interaction of drugs with human serum albumin (HSA) was developed. The methodology was based on the quenching of the intrinsic fluorescence of HSA by binding of the drug to one of its binding sites. The fluorescence quenching assay was implemented in a sequential injection analysis (SIA) system and the optimized assay was applied to ionic liquids based on the association of non-steroidal anti-inflammatory drugs with choline (IL-API).
In each cycle, 100 µL of HSA and 100 µL of IL-API (variable concentration) were aspirated at a flow rate of 1 mL min−1 and then sent through the reaction coil to the detector where the fluorescence intensity was measured.
In the optimized conditions the effect of increasing concentrations of choline ketoprofenate and choline naproxenate (and respective starting materials: ketoprofen and naproxen) on the intrinsic fluorescence of HSA was studied and the dissociation constants (Kd) were calculated by means of models of drug–protein binding in the equilibrium. The calculated Kd showed that all the compounds bind strongly to HSA (Kd<100 µmol L−1) and that the use of the drugs in the IL format does not affect or can even improve their HSA binding.
The obtained results were compared with those provided by a conventional batch assay and the relative errors were lower than 4.5%. The developed SIA methodology showed to be robust and exhibited good repeatability in all the assay conditions (rsd<6.5%).
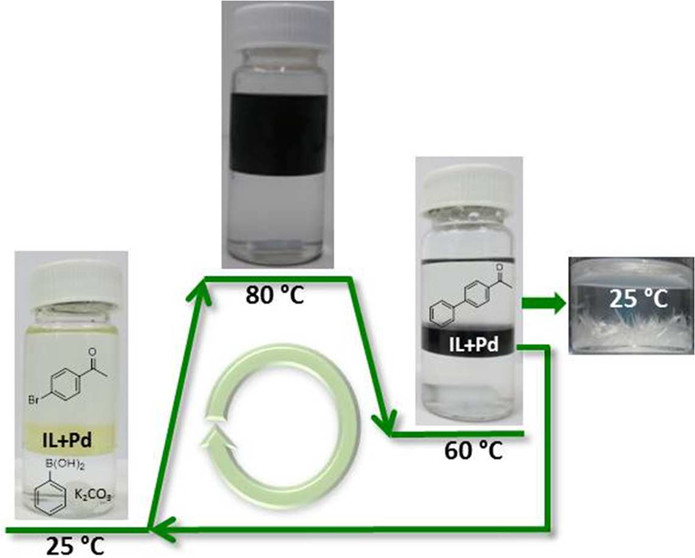
Ionic liquid-based microemulsions in catalysis
Int. J. Org. Chem. (2016) 81, 12332
The design and properties of surface-active ionic liquids that are able to form stable microemulsions with heptane and water are presented, and their promise as reaction media for thermomorphic palladium-catalyzed cross-coupling reactions is demonstrated.
Synthesis of Partially Deuterated N-Nitrosamines – New Standards in Tobacco-smoke Analysis
Peter Gaertner*, Katharina Bica, Christian Einzinger
Monatsh. Chem. (2004) 135, 549
N-Nitrosamines of tobacco alkaloids contribute to the cause of tobacco related cancers and, therefore, they are important analytes. Herein the preparation of four partially deuterated N-nitrosamines from simple, commercially available, deuterated educts is described, which can serve as useful standards in tobacco-smoke analysis.
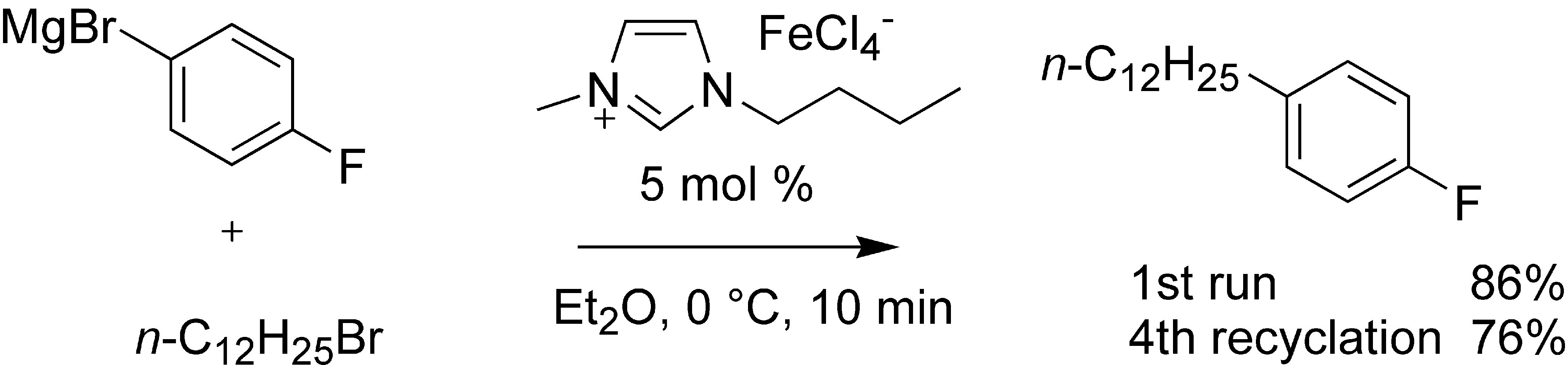
An Iron-Containing Ionic Liquid as Recyclable Catalyst for Aryl Grignard Cross-Coupling of Alkyl Halides
Org. Lett. (2006) 8, 733
The ionic liquid butylmethylimidazolium tetrachloroferrate (bmim-FeCl4) was found to be a very effective and completely air stable catalyst for the biphasic Grignard cross-coupling with primary and secondary alkyl halides bearing β-hydrogens. After simply decanting the product in the ethereal layer, the ionic liquid catalyst was successfully recycled four times.
Synthesis and analytics of 2,2,3,4,4-d5-19-nor-5α-androsterone—An internal standard in doping analysis
Steroids (2007) 72, 429
A short and efficient synthesis of pentadeuterated 2,2,3,4,4-d5-19-nor-5α-androsterone 7 starting from 19-norandrost-4-ene-3,17-dione 1 by a d1-l-Selectride mediated stereo- and regioselective reduction of the 3-keto group is presented. The use of compound 7 as internal standard for the detection of anabolic steroids via mass spectrometric techniques such as gas chromatography–mass spectrometry (GC–MS) is discussed.
Microwave-Assisted Synthesis of Camphor-Derived Chiral Imidazolium Ionic Liquids and Their Application in Diastereoselective Diels–Alder Reaction
Synthesis. (2007) 9, 1333
Starting from cheap chiral pool precursors camphorsulfonic acid and camphene, several imidazolium ionic liquids were synthesized in high overall yield. Both thermal and microwave-assisted synthesis was used in the quaternization step to obtain new chiral ionic liquids (CILs) bearing a bornyl structural motif as cation. Furthermore, these CILs were used as solvent in the Diels-Alder reaction of acrylic acid and cyclopentadiene and showed good yields and diastereoselectivities.
Applications of Chiral Ionic Liquids
Eur. J. Org. Chem. (2008) 2008, 3235
Despite the rapid growth in chiral ionic liquid (CIL) research, successful applications remained hidden for some time, but are increasing fast nowadays. The major part of this review deals with the use of CILs as solvents, catalysts or ligands in asymmetric synthesis. Chiral recognition abilities in spectroscopic techniques such as NMR, NIR or fluorescence spectroscopy are discussed as well as the application of CILs for separation in chromatography. In this review, we focus entirely on the applications of CILs; publications dealing with design and synthesis of CILs only are not mentioned.
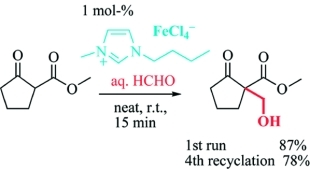
Metal-Containing Ionic Liquids as Efficient Catalysts for Hydroxymethylation in Water
Eur. J. Org. Chem. (2008) 2008, 3453
The iron-containing ionic liquid butylmethylimidazolium tetrachloroferrate (bmim-FeCl4) proved to be an efficient and recyclable catalyst for the hydroxymethylation of β-keto esters using aqueous formaldehyde and a low catalyst loading of up to 0.1 mol-% without co-solvents or additional surfactants. An useful and high-yielding approach to hydroxymethylated keto esters as well as to 3-disubstitued butyrolactones via tandem hydroxymethylation–lactonization could thus be established.
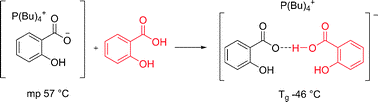
Confused ionic liquid ions—a “liquification” and dosage strategy for pharmaceutically active salts
Chem. Commun. (2010) 46, 1215
We present a strategy to expand the liquid and compositional ranges of ionic liquids, specifically pharmaceutically active ionic liquids, by simple mixing with a solid acid or base to form oligomeric ions.
In search of pure liquid salt forms of aspirin: ionic liquid approaches with acetylsalicylic acid and salicylic acid
Phys. Chem. Chem. Phys. (2010) 12, 2011
We present an ionic liquid (IL) approach towards a dual functional liquid salt form of aspirin using different pharmaceutically active cations composed of antibacterials, analgesics, local anesthetics, and antiarrhythmic drugs in combination with acetylsalicylic acid or its metabolite salicylic acid and discuss stability of these ILs in comparison to solid salts. Several low-melting or liquid salts of salicylic acid with dual functionality and promising properties were isolated and characterized; however, although such ILs with aspirin could be prepared, they suffer from limited stability and slowly decompose into the corresponding salicylate ILs when exposed to moisture.
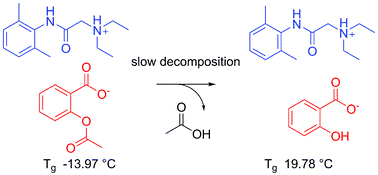
Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper
Pharm. Res. (2010) 27, 521
Why not consider liquid salt forms of active pharmaceutical ingredients (APIs) as an alternative versatile tool in the pharmaceutical industry? Recent developments have shown that known APIs can be readily converted into ionic liquids and that these novel phases often possess different properties (e.g., improved solubilities and dissolution rates), which may have a direct impact on the pharmacokinetics and pharmacodynamics of the drug. They may also offer the potential of novel and more efficient delivery modes, as well as patent protection for each of the new forms of the drug. Since these pharmaceutically active ionic liquids represent a thermodynamically stable phase, they avoid the troublesome issues surrounding polymorphism and “polymorphic transformation.” In some cases, an active cation and an active anion can be combined to produce a liquid possessing dual functionality. Here we examine and challenge the current industry reliance on crystalline APIs by discussing the breadth and potential impact of liquid salts as a possible approach to phase control.
Application of meso-hydrobenzoin-derived chiral auxiliaries for the stereoselective synthesis of highly substituted pyrrolidines by 1,3-dipolar cycloaddition of azomethine ylides
Tetrahedron Asymmetry (2010) 21, 641
The metal-catalyzed stereoselective 1,3-dipolar cycloaddition of azomethine ylides and acrylates using recyclable meso-hydrobenzoin-derived chiral auxiliaries is described. Cleavage of the auxiliary leads to highly substituted pyrrolidines in up to 87% enantiomeric excess.
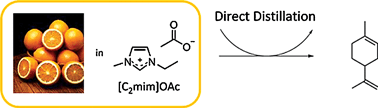
Ionic liquids and fragrances – direct isolation of orange essential oil
Green Chem. (2011) 13, 1997
We present the dissolution of fresh fragrance biomass in ionic liquids for the isolation of essential oils and compare direct distillation with solvent extraction. Orange essential oil was distilled from orange peels dissolved in ionic liquid media, thus allowing a simple, efficient, and mild isolation of fragrance and flavor components with complete recovery of the ionic liquid.
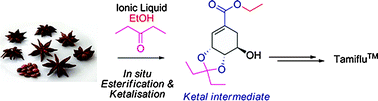
From plant to drug: ionic liquids for the reactive dissolution of biomass
Green Chem. (2011) 13, 1442
We present an ionic liquid (IL) strategy for the reactive dissolution of star anise seeds using different Brønsted-acidic ionic liquids as the solvent and reaction media towards the isolation of important pharmaceutical intermediates; this procedure provides a single-step, higher yielding and environmentally benign strategy towards the synthesis of the anti-influenza drug Tamiflu™.
Toxic on purpose: ionic liquidfungicides as combinatorial crop protecting agents
Green Chem. (2011) 13, 2344
We present an ionic liquid strategy for novel hydrophobic fungicide forms of the actives thiabendazole and imazalil with increased rain persistence and activity against potato tuber diseases.
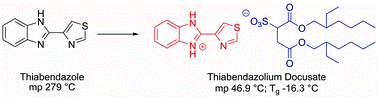
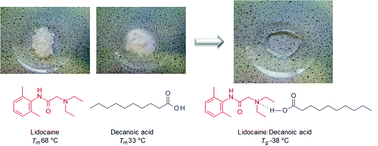
Liquid forms of pharmaceutical co-crystals: exploring the boundaries of salt formation
Chem. Commun. (2011) 47, 2267
We present evidence of hydrogen bond formation, not salt formation, as the driving force in the liquefaction of a solid pharmaceutical in the form of a neutral acid–base complex, as exemplified by the liquid formed from a mixture of the local anesthetic lidocaine with fatty acids; these complexes exist at the boundary between simple eutectics and partially ionised ionic liquids.
From Solvent to Sustainable Catalysis – Chloroferrate Ionic Liquids in Synthesis
Curr. Org. Synth. (2011) 8, 824
During the past few years, Lewis-acidic ionic liquids (ILs) have emerged from their role as mere reaction media toward a role as novel catalytic systems. Iron-containing chloroferrate ionic liquids in particular offer a novel approach to sustainable and environmentally benign catalysis and can be applied in a surprising diversity of transformations. In this review, we present this emerging topic and report the application of chloroferrate ILs as solvents, catalysts or catalytic systems for a variety of chemical processes, with a particular focus on reactions leading to pharmaceutically active compounds or intermediates.
Pharmaceutically active ionic liquids with solids handling, enhanced thermal stability, and fast release
Chem. Commun. (2012) 48, 5422
Pharmaceutically active compounds in ionic liquid form immobilized onto mesoporous silica are stable, easily handled solids, with fast and complete release from the carrier material when placed into an aqueous environment. Depending on specific ion-surface interactions, they may also exhibit improved thermal stability when compared to the non-adsorbed compounds.
Micellar catalysis in aqueous–ionic liquid systems
Chem. Commun. (2012) 48, 5013
We present the application of ionic liquid–aqueous micellar solutions as reaction media for Diels–Alder reaction and found that reaction rates could be significantly increased compared to the reaction in water.
New aspects for biomass processing with ionic liquids: towards the isolation of pharmaceutically active betulin
Green Chem. (2012) 14, 940
By utilising ionic liquids the pharmaceutically active triterpene betulin can be extracted from biomass with significantly improved extraction yield and purity. The recovery of the ionic liquid 1-ethyl-3-methylimidazolium acetate via azeotropic distillation of EtOH/H2O was successfully demonstrated.

Iron catalyzed Michael addition: Chloroferrate ionic liquids as efficient catalysts under microwave conditions
Sci. China Chem. (2012) 55, 1614
The iron containing ionic liquid 1-butyl-3-methylimidazolium tetrachloroferrate ([C4mim]Cl/FeCl3; χ = 0.5) proved to be an efficient catalyst for the solvent-free metal catalyzed Michael addition of β-keto esters and enones. Due to the ionic structure of the catalyst, a significant acceleration of the reaction rate was observed under microwave conditions. Furthermore, recycling of the ionic liquid catalyst could be performed after simply distilling off the products.
Active pharmaceutical ingredients based on salicylate ionic liquids: insights into the evaluation of pharmaceutical profiles
New J. Chem. (2013) 37, 4095
This work is based on the evaluation of the protein binding affinity, partition coefficients (with a biomimetic membrane) and surfactant properties of three pharmaceutically active ionic liquids based on the salicylate anion. Fluorescence spectroscopy was used for the evaluation of the binding of ionic liquids to human serum albumin and for the determination of critical micelle concentrations. Partition coefficients were determined using micelles of hexadecylphosphocholine and UV-Vis derivative spectroscopy. The results indicate that all the compounds bind strongly and spontaneously to human serum albumin and exhibit the ability to form micelles. The determined partition coefficients were up to 6 times higher than those of the starting materials, evidencing that the ionic liquid form has greater affinity for the lipid phase than the inorganic salt form of salicylate. Generally, the studied salicylate ionic liquids exhibit an interesting pharmaceutical profile presenting favorable properties regarding the incorporation of the compounds in antimicrobial pharmaceutical formulations. It was evidenced that the tested ionic liquids can exert direct effects on cell membranes as indicated by their surfactant properties and high ability to partition to hydrophobic environments.
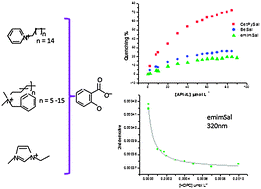
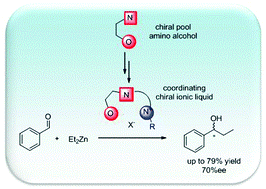
Coordinating chiral ionic liquids
Org. Biomol. Chem. (2013) 11, 8092
A practical synthesis of novel coordinating chiral ionic liquids with an amino alcohol structural motif was developed starting from commercially available amino alcohols. These basic chiral ionic liquids could be successfully applied as catalysts in the asymmetric alkylation of aldehydes and gave high enantioselectivities of up to 91% ee.
Prodrug ionic liquids: functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form
Med. Chem. Commun. (2013) 4, 559
Neutral, non- or not easily-ionizable active pharmaceutical ingredients can take advantage of the unique property sets of ionic liquids by functionalization with hydrolyzable, charged (or ionizable) groups in the preparation of ionic liquid prodrugs as demonstrated here with the synthesis, characterization, and hydrolysis of cationic acetaminophen prodrugs paired with the docusate anion.
Polarisabilities of alkylimidazolium ionic liquids
Phys. Chem. Chem. Phys. (2013) 15, 2703
The molar polarisability and molar volume for 71 ionic liquids were extracted from 157 measurements of their refractive index and density, which were then further deconstructed into atomic contributions by means of a Designed Regression analysis. Using this approach, the density and refractive index for any chosen ionic liquid with alkyl-substituted imidazolium cations can be predicted in good agreement with experimental data.
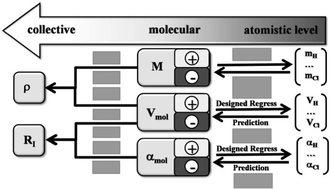
Exploring ionic liquid–biomass interactions: towards the improved isolation of shikimic acid from star anise pods
RSC Adv. (2013) 3, 26010
Based on shikimic acid – the starting material for the important anti-influenza drug Tamiflu (oseltamivir phosphate) – we present that the dissolution properties of ionic liquids can lead to better access to the valuable ingredient embedded in the biopolymer. Different imidazolium-based ionic liquids were investigated, and the extraction yield of shikimic acid was correlated with their hydrogen-bonding properties via polarizable MD simulations, indicating that the hydrogen bonding of the IL anion to shikimic acid is responsible for a good extraction performance. A scale-up strategy for the isolation of shikimic acid with the ionic liquid 1-ethyl-3-methylimidazolium acetate ([C2mim]OAc) was developed based on ion-exchange resins, thus allowing isolation of shikimic acid in up to 10 wt% yield with complete recovery of the ionic liquid.
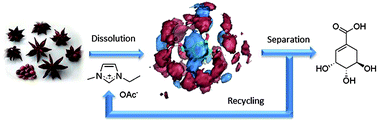
Basic chiral ionic liquids: A novel strategy for acid-free organocatalysis
Catal. Today (2013) 200, 80
We present design, synthesis and application of basic chiral ionic liquids based on commercially available (S)-proline. This new set of chiral ionic liquids was specifically designed to replace trifluoroacetic acid in enamine-based organocatalysis for asymmetric Csingle bondC bond formation. Based on their permanent charge, these chiral ionic liquids could be applied as organocatalysts in asymmetric aldol reaction of 4-nitrobenzaldehyde and acetone, and good yields and selectivities up to 80%ee could be obtained without additional acid.
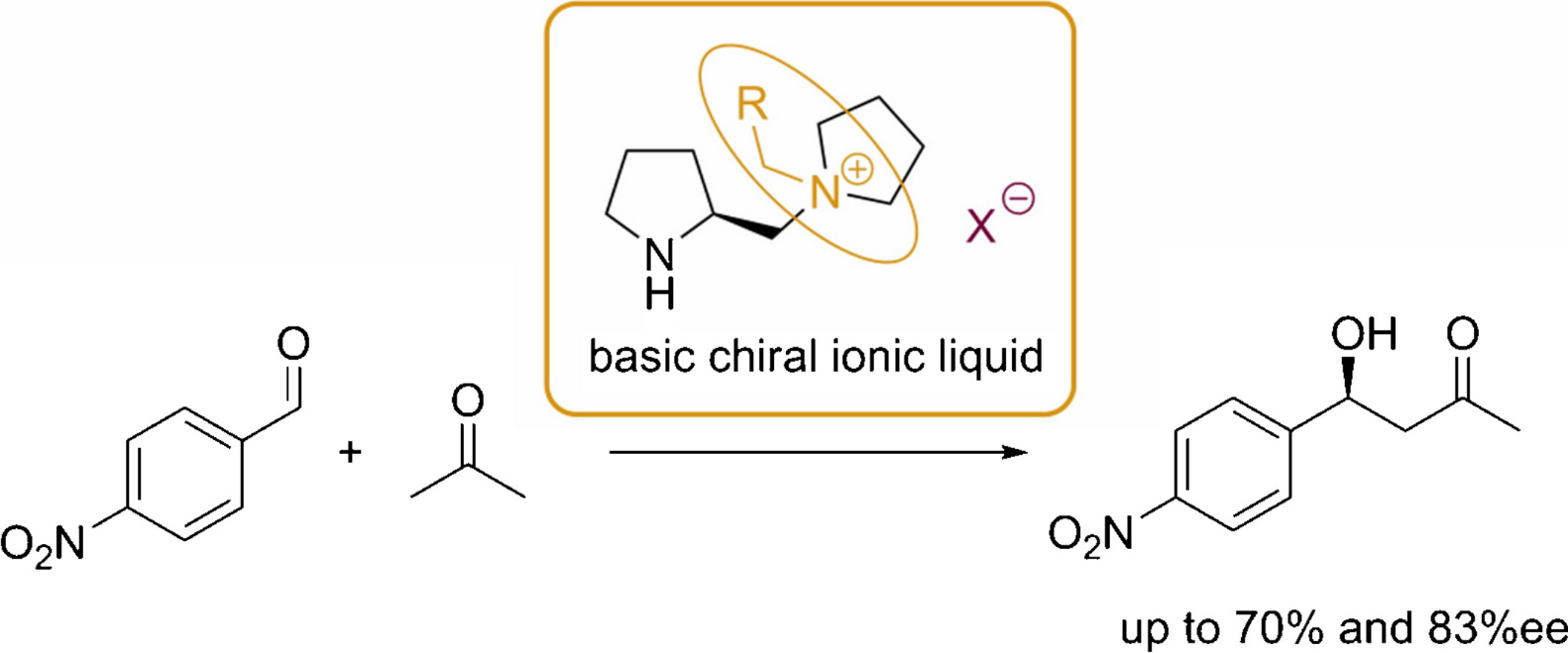
Design, synthesis, and application of novel chiral ONN ligands for asymmetric alkylation
Monatsh. Chem. (2013) 144, 447
Starting from easily available chiral pool amino alcohols, a set of novel chiral ONN ligands was synthesized and their catalytic potential was examined in asymmetric alkylation. Ligands with a secondary central NH group and N-methylated ONN ligands were successfully applied as catalysts in the addition of diethyl zinc to various aromatic aldehydes, yielding secondary alcohols in excellent yields of up to 99 % ee.
Surface-active Ionic Liquids for Micellar Extraction of Piperine from Black Pepper
Z. Naturforsch. B (2013) 68b, 1129
We present the application of ionic liquid-aqueous micellar solutions as isolation media for the pharmaceutically active ingredient piperine from black pepper. Several surface-active ionic liquids including a biodegradable betaine derivative were used for the extraction of piperine, and a strong correlation between extraction yield and the critical micelle concentration of the respective ionic liquid was found. A scaled strategy for the isolation of piperine was developed that allowed recovery and recycling of the aqueous ionic liquid micellar solution for five runs without any loss in extraction efficiency
Automated evaluation of pharmaceutically active ionic liquids’ (eco)toxicity through the inhibition of human carboxylesterase and Vibrio fischeri
J. Hazard. Mater. (2014) 265, 133
The toxicity of 16 pharmaceutical active ionic liquids (IL-APIs) was evaluated by automated approaches based on sequential injection analysis (SIA). The implemented bioassays were centered on the inhibition of human carboxylesterase 2 and Vibrio fischeri, in the presence of the tested compounds. The inhibitory effects were quantified by calculating the inhibitor concentration required to cause 50% of inhibition (EC50). The EC50 values demonstrated that the cetylpyridinium group was one of the most toxic cations and that the imidazolium group was the less toxic. The obtained results provide important information about the safety of the studied IL-APIs and their possible use as pharmaceutical drugs. The developed automated SIA methodologies are robust screening bioassays, and can be used as a generic tool to identify the (eco)toxicity of the structural elements of ILs, contributing to a sustainable development of drugs.
Direct extraction of genomic DNA from maize with aqueous ionic liquid buffer systems for applications in genetically modified organisms analysis
Anal. Bioanal. Chem. (2014) 406, 7773
To date, the extraction of genomic DNA is considered a bottleneck in the process of genetically modified organisms (GMOs) detection. Conventional DNA isolation methods are associated with long extraction times and multiple pipetting and centrifugation steps, which makes the entire procedure not only tedious and complicated but also prone to sample cross-contamination. In recent times, ionic liquids have emerged as innovative solvents for biomass processing, due to their outstanding properties for dissolution of biomass and biopolymers. In this study, a novel, easily applicable, and time-efficient method for the direct extraction of genomic DNA from biomass based on aqueous-ionic liquid solutions was developed. The straightforward protocol relies on extraction of maize in a 10 % solution of ionic liquids in aqueous phosphate buffer for 5 min at room temperature, followed by a denaturation step at 95 °C for 10 min and a simple filtration to remove residual biopolymers. A set of 22 ionic liquids was tested in a buffer system and 1-ethyl-3-methylimidazolium dimethylphosphate, as well as the environmentally benign choline formate, were identified as ideal candidates. With this strategy, the quality of the genomic DNA extracted was significantly improved and the extraction protocol was notably simplified compared with a well-established method.
