Latest Publications

About Katharina Schröder
Prof. Katharina Schröder (nee Bica) was born in Vienna, Austria in 1980. After receiving her PhD degree in Technical Chemistry from TU Wien, Dr. Schröder accepted a position as research fellow, working with leading experts in the field of Green Chemistry and Catalysis at The Queen’s University Belfast, UK and the Technical University Denmark in Lyngby, DK. After returning to TU Wien in October 2009 for a tenured position, she established a new research group focused on sustainable chemistry through innovation with alternative solvents. She was promoted to associate professor in 2018 and to full professor for Sustainable Chemistry in 2020. At the moment, she is leading or is involved in several other national and EU-funded projects, including an ERC Consolidator Grant 2019.
Katharina Schröders research interests are based on sustainable organic chemistry, with a special focus on (i) organo- and photocatalysis for asymmetric synthesis, (ii) on carbon capture and valorization techniques (CCU), particularly on photocatalytic CO2 activation and (iii) on the recovery of valuable ingredients from industrial waste streams using advanced fluid technologies.
Researcher profile
ORCID iD: 0000-0002-2515-9873
Researchgate Profile: https://www.researchgate.net/profile/Katharina_Bica
Honors & Awards
- ERC Consolidator Grant 2019 (CARBOFLOW: Streamlined CO2 conversion in ionic liquids – a platform strategy for modern carbonylation chemistry)
- Best teaching award 2017 (“Organic Chemistry”, together with M.D. Mihovilovic, F. Rudroff and M. Schnürch)
- FILL Symposium Lecture Award 2014, Tokyo, Japan
- INITS-Award 2012 (3rd place category “General technologies”)
- Poster awards at Green Solvents for Synthesis; Boppard, Germany, 10/2012; Green Solvents for Processes; Friedrichshafen,
- Germany, 10/2006; 2nd International Congress on Ionic Liquids; Yokohama, Japan, 08/2007
- Prize of the Industrial Advisory Board; QUILL Meeting, Belfast, UK, 03/2009
- ASAG – award for scientific research abroad; 05/2007
- Award for outstanding diploma thesis of the city of Vienna; 04/2005
Honors & Awards
- Chair and Organizer of the 26th EUCHEM Conference on Molten Salts and Ionic Liquids; Vienna, Austria, 2016.
- COST Action CM1206: Work Group Leader “Applications of Ionic Liquids” since 05/2013
- COST Action CM1206: Member of the Management Committee since 05/2013
- Faculty Board Member (“Fakultätsrat”), Faculty of Technical Chemistry, TU Wien
- Member of the committee of study affairs of Technical Chemistry (“Studienkommission Technische Chemie”), TU Wien
Teaching
Our group is strongly involved in teaching for under- and postgraduate students at TU Wien, covering lectures and laboratories from the fundamentals of chemistry towards advanced organic chemistry. Apart from teaching lectures in the fields of asymmetric synthesis, organocatalysis or green chemistry, Katharina Schröder is substantially involved in the development of the novel Green Chemistry Master program, a newly established MSc program Green Chemistry that provides hands-on learning opportunities for students to develop innovative solutions for more sustainable processes.
Additionally, our team members are supervising laboratory classes on (advanced) organic chemistry, fundamentals of chemistry or the newly established laboratory class on green chemistry, and undergraduate students can frequently join our group via short internships or Bachelor research projects to get in contact with our active research topics.
Grants
Ongoing Projects

CARBOFLOW: Streamlined CO2 conversion – a platform strategy for modern carbonylation chemistry
5 year (2021-2026) ERC Consolidator project funded by the European Research Council

CO2 Refinery
4 year (2020-2024) doctoral college funded by TU Wien

Halide-free synthesis of novel fluorinated ionic liquids
4 year (2020-2024) stand-alone project funded by the FWF (P29146)

PLATIRUS: PLATInum group metals Recovery Using Secondary raw materials
5 year collaborative Project H2020-SC5-2016-2017 (2016-2023) funded by the European Commission (730224)
Past Projects

Self-aggregation of chiral ionic liquids and its impact on asymmetric catalysis
5 year (2016-2022) stand-alone project funded by the FWF (P29146)

Surface science and model catalysis of supported ionic liquids
3 year (2019-2022) innovative project funded by TU Wien

Ionic Liquid-Aqueous Micellar Systems for Sustainable Catalysis
3 year (2013-2017) stand-alone project funded by the FWF (P25504)

minIONs: Minimalschmierbeschichtung auf Basis polymerisierter ionischer Flüssigkeiten
2 year (2017-2019) cooperative project funded by the FFG (859784)

Kontinuierliche Produktion wertvoller Chemikalien aus Kohlendioxid – Ein Weg zur Aufwertung von CO2
2 year (2016-2018) project funded by the Hochschuljubiläumsstiftung der Stadt Wien” (H286949/2016)

CObalt and LAnthanide recovery from BATerieS (COLABATS)
3 year collaborative project FP7-ENV-2013-6.3-1 (2013-2016) funded by the European Commission (603482)

Chiral Ionic Liquids in Separation Sciences
3 year (2010-2013) innovative project funded by TU Wien

Active Ingredient Isolation with Ionic Liquids
2 year (2010-2012) project funded by the Hochschuljubiläumsstiftung der Stadt Wien” (H2121/2010)

Optimierte Schienenkopf-Konditionierung durch alternative Konditioniermittel, wie Ionische Flüssigkeiten, und Aufbringungsapparaturen
2 year (2009-2011) project funded by the FFG (P820731), together with J. Fröhlich

Chiral and Metal Containing Ionic Liquids
2 year (2007-2009) project funded by the Hochschuljubiläumsstiftung der Stadt Wien” (H1681/2006)
Student Grants

Active Ingredient Isolation with Ionic Liquids
3 year (2012- 2015) student grant for A. K. Ressmann funded by the Austrian Academy of Sciences (DOC-fFORTE 234567)

Active Ingredient Isolation with Ionic Liquids
11 month (June 2011- April 2012) student grant for K. Strassl funded by TU Wien

Micellar Catalysis
9 month (July 2011-March 2012) student grant for A. K. Ressmann funded by TU Wien
Photocatalyst-free hydroacylations of electron-poor alkenes and enones under visible-light irradiation
Org. Biomol. Chem. (2022) 20, 7245
Herein we present a photocatalyst- and additive-free radical hydroacylation of electron-poor double bonds under mild reaction conditions. Using 4-acyl-Hantzsch ester radical reservoirs, various Michael acceptors, enones and para-quinone methide substrates could be used. The protocol enabled further derivatizations and it could also be extended to a few unactivated alkenes. Moreover, the nature of the radical process was also investigated.

Surface-active ionic liquids: A review
J. Mol. Liq. (2022) 347, 118160
The combination of water and surface-active ionic liquids provides a unique reaction medium, facilitating aggregation and micellization of the ionic liquid to allow for chemical reactions in bulk water. With a growing focus on sustainable technologies, ionic liquids have emerged as tunable solvents for multiple applications but are often too viscous or expensive for use as bulk solvent. As a result, there has been a tremendous increase in interest in the behavior of ionic liquids in the presence of water. It has already been shown that certain ionic liquids act as surfactants in aqueous solutions, enabling the combination of both solvents to afford solvent systems with unique properties. Ultimately, surface-active ionic liquids in water give rise to distinct chemistry of their own compared to traditional molecular solvents, and thus their use is rapidly growing. In this review, the general structure of surface-active ionic liquids and the key features that allow aggregation in water to give micellar structures is discussed. Furthermore, characterization techniques of the formed micelles are presented, discussing aggregation and possible methods of studying micellization behavior. Finally, current applications of surface-active ionic liquids across all fields of chemistry, from traditional organic chemistry to nanoparticle synthesis are presented.

Liquid- and Solid-based Separations Employing Ionic Liquids for the Recovery of Platinum Group Metals Typically Encountered in Catalytic Converters: A Review
ChemSusChem (2022) 15, e202102262
The wide application range and ascending demand for platinum group metals combined with the progressive depletion of their natural resources renders their efficient recycling a very important and pressing matter. Primarily environmental considerations associated with state-of-the-art recovery processes have shifted the focus of the scientific community toward the investigation of alternative recycling approaches. Within this context, ionic liquids have gained considerable attention in the last two decades chiefly sparked by properties such as tunabilty, low-volatility, and relatively easy recyclability. In this review an understanding of the state-of-the-art processes, including their drawbacks and limitations, is provided. The core of the discussion is focused on platinum group metal recovery with ionic liquid-based systems. A brief insight in some environmental considerations related to ionic liquids is also provided while some discussion on research gaps, common misconceptions related to ionic liquids and outlook on unresolved issues could not be absent from this review.
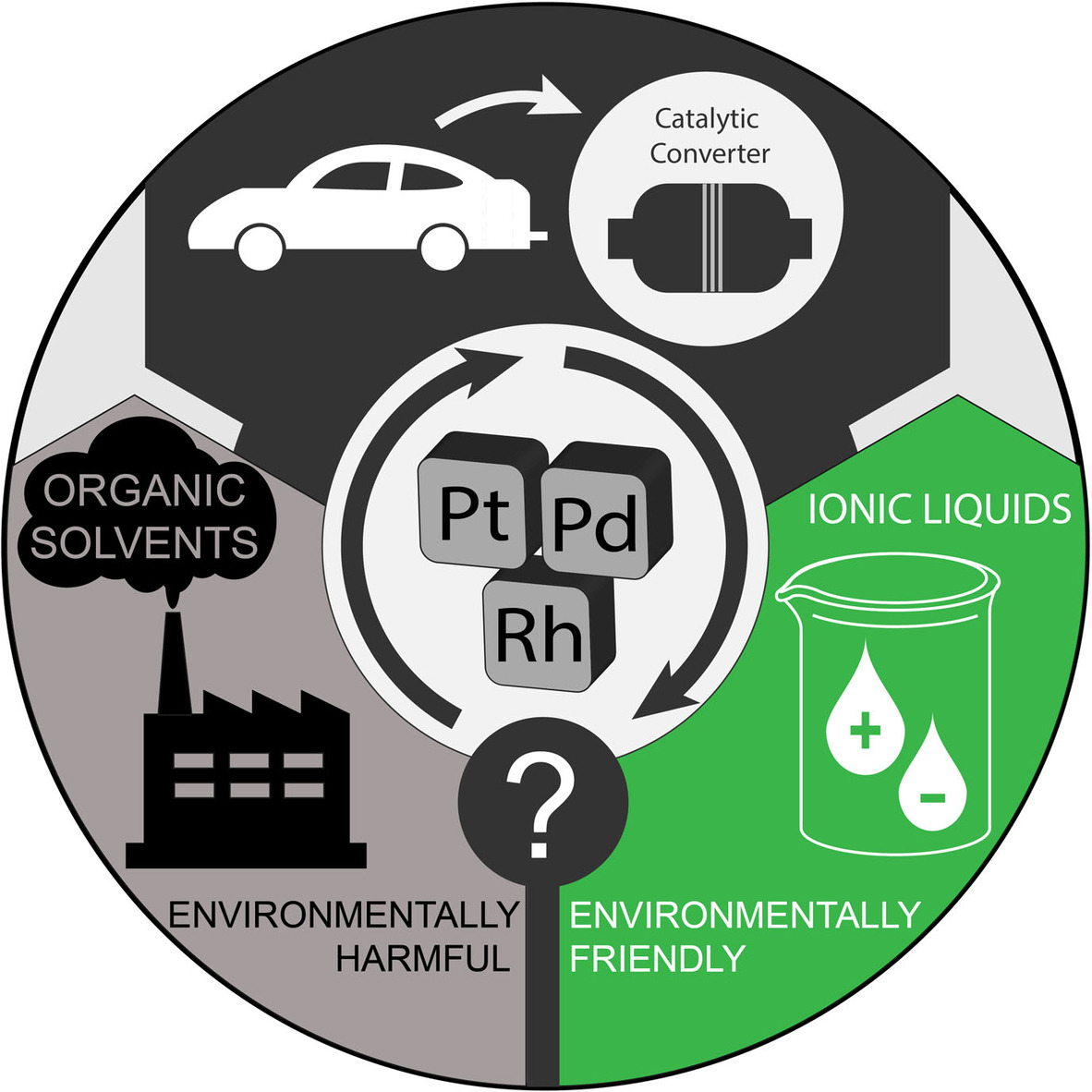
Benign recovery of platinum group metals from spent automotive catalysts using choline-based deep eutectic solvents
Green Chem. Lett. Rev. (2022) 15, 405
The recovery of platinum group metals (PGMs) from secondary raw materials has become a topic ofcritical importance mainly due to the gradual depletion of their natural resources and theircontinuously increasing demand. However, the insufficient recovery of PGMs coupled with thenegative environmental impact of the state-of-the-art recycling procedures mandate theinvestigation and development of alternative recovery processes that will assist in minimizing oreven eliminating these drawbacks. Herein, we present a process for the extraction of platinumgroup metals from spent car catalysts relying on benign deep eutectic solvents (DESs). It isdemonstrated that with addition of small amounts of an oxidizing agent, deep eutectic solventscan act as excellent leaching media for the quantitative extraction of platinum group metals.Despite its inertness towards acidic and oxidizing agents, Rh can be leached in a considerableamount which can be further increased by physical pre-treatment of the spent car catalystmaterial.

Sterically Demanding Flexible Phosphoric Acids for Constructing Efficient and Multi-Purpose Asymmetric Organocatalysts
Angew. Chem. Int. Ed. (2022) 61, e202202189
Herein, we present a novel approach for various asymmetric transformations of cyclic enones. The combination of readily accessible chiral diamines and sterically demanding flexible phosphoric acids resulted in a simple and highly tunable catalyst framework. The careful optimization of the catalyst components led to the identification of a particularly powerful and multi-purpose organocatalyst, which was successfully applied for asymmetric epoxidations, aziridinations, aza-Michael-initiated cyclizations, as well as for a novel Robinson-like Michael-initiated ring closure/aldol cyclization. High catalytic activities and excellent stereocontrol was observed for all four reaction types, indicating the excellent versatility of our catalytic system. Furthermore, a simple change in the diamine’s configuration provided easy access to both product antipodes in all cases.

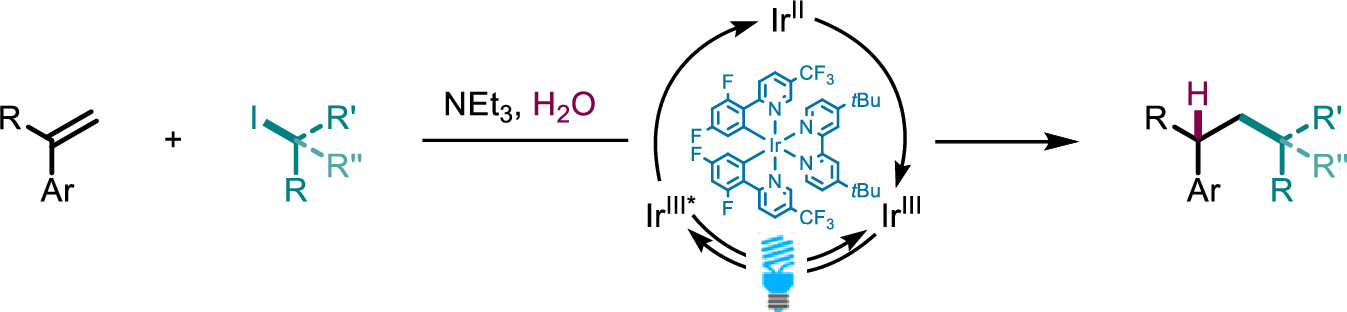
Photocatalytic Hydroalkylation of Aryl-Alkenes
J. Org. Chem. (2022) 87, 11042
Here, we present a visible light-catalyzed hydroalkylation of aryl-alkenes affording C–C bonds using aryl-alkenes and alkyl iodides. We demonstrate the formation of various hydroalkylation products in excellent yields, with primary, secondary, and tertiary alkyl iodides being tolerated in the reaction. Mechanistic experiments reveal a pathway consisting of halogen atom transfer followed by a radical-polar crossover mechanism delivering the desired hydroalkylation products.
Halide-Free Continuous Synthesis of Hydrophobic Ionic Liquids
ACS Sustainable Chem. Eng. (2022) 10, 11215
Herein, we present a novel approach for the halide-free, continuous-flow preparation of hydrophobic ionic liquids (ILs) relying on the bis(trifluoromethanesulfonyl)imide (bistriflimide, NTf2–) anion. The simple yet fast two-step synthetic route, which involves the formation of different alkyl bistriflimides (R4NTf2), followed by the quaternization with an amine nucleophile, led to the desired ILs in high yields and excellent purities without any byproduct formation. The variable alkyl chain (R4) length and the broad range of the applicable nucleophiles (R1R2R3N) offer considerable flexibility to the synthetic protocol. The quaternization can be performed under solvent-free conditions; moreover, the homogeneous nature of these reactions allows the application of modern continuous-flow technologies. Given these advantages, the methodology can afford not just a fast and efficient alternative for the conventional synthesis of such compounds with reduced waste water production but their negligible halide content might provide a significantly broader application range of the IL products, especially for the field of materials science.

Continuous Formation of Limonene Carbonates in Supercritical Carbon Dioxide
Philipp Mikšovsky, Elias N. Horn, Shaghayegh Naghdi, Dominik Eder, Michael Schnürch, Katharina Bica-Schröder*
Org. Process Res. Dev. (2022) 26, 2799
We present a continuous flow method for the conversion of bioderived limonene oxide and limonene dioxide to limonene carbonates using carbon dioxide in its supercritical state as a reagent and sole solvent. Various ammonium- and imidazolium-based ionic liquids were initially investigated in batch mode. For applying the best-performing and selective catalyst tetrabutylammonium chloride in continuous flow, the ionic liquid was physisorbed on mesoporous silica. In addition to the analysis of surface area and pore size distribution of the best-performing supported ionic liquid phase (SILP) catalysts via nitrogen physisorption, SILPs were characterized by diffuse reflectance infrared Fourier transform spectroscopy and thermogravimetric analysis and served as heterogeneous catalysts in continuous flow. Initially, the continuous flow conversion was optimized in short-term experiments resulting in the desired constant product outputs. Under these conditions, the long-term behavior of the SILP system was studied for a period of 48 h; no leaching of catalyst from the supporting material was observed in the case of limonene oxide and resulted in a yield of 16%. For limonene dioxide, just traces of leached catalysts were detected after reducing the catalyst loading from 30 to 15 wt %, thus enabling a constant product output in 17% yield over time.

