
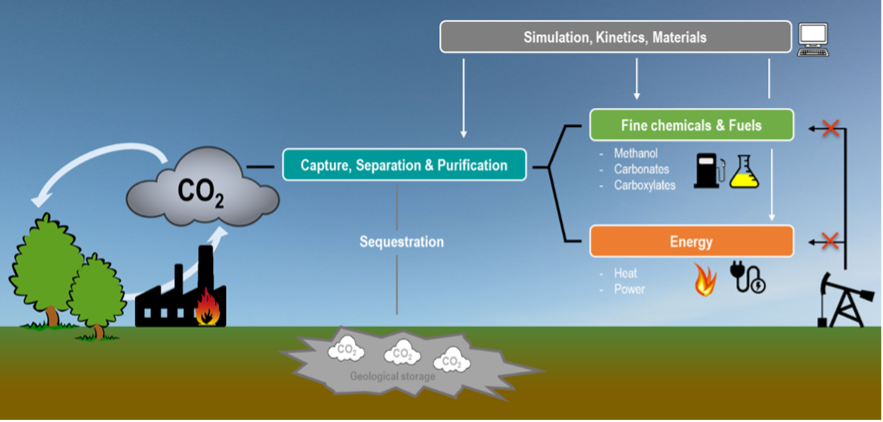
Carbon dioxide from the combustion of fossil sources is regarded as the most significant greenhouse gas; hence, CO2 capture and conversion has attracted considerable attention in the past years. As an abundant, nontoxic, non-flammable and renewable carbon resource, CO2 is attractive as a feedstock for making fine chemicals and materials.
Supercritical CO2: Combining solvent and reagent
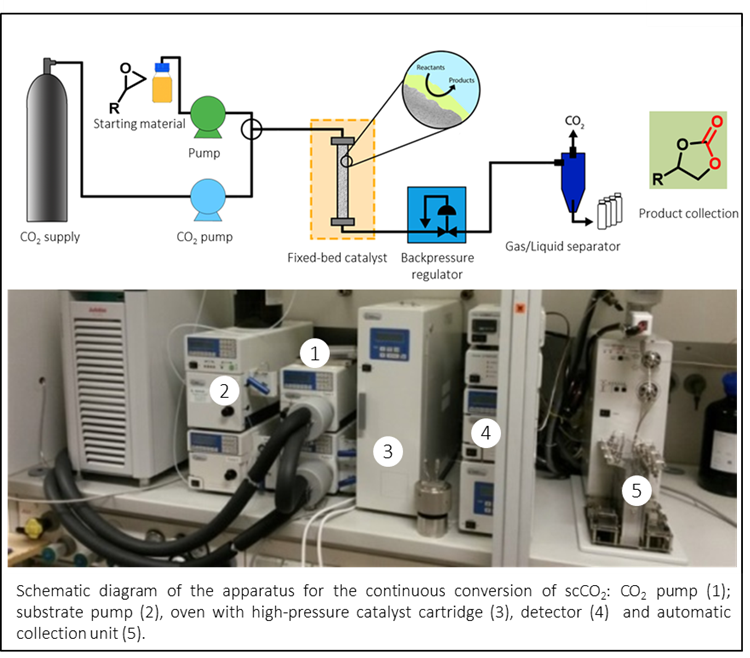
Supercritical carbon dioxide (scCO2) has been shown to serve as an environmentally benign substitute for a number of solvents, for example in the industrial decaffeination of coffee beans. Starting with the continuous synthesis of propylene carbonate used as electrolyte in lithium ion batteries, we became interested in the synthesis of biscarbonates derived from biorenewables such as terpenes, oils or fats and scCO2, ultimately aiming for the sustainable production of isocyanate-free polyurethanes. Heterogeneous catalysts based on supported or polymerized catalysts were developed, thus allowing a continuous conversion of scCO2 used simultaneously as solvent and carbon source.
Membrane reactors for CO2 refinery
Within the doctoral school CO2 Refinery we are working on catalytically active polymeric hollow-fibre membrane reactor, with direct applicability for low-temperature CO2 conversion from crude gas. Hollow-fibre membranes are fabricated in house and modified with catalytically active species. Through the optimization of the spinning process, we aim for an asymmetric, microporous hollow‐fibres, and ultimately for the development of a membrane reactor with high surface area. Eventually, this strategy provides a tool for immobilizing catalysts while simultaneously overcoming equilibrium constraints.
