Rudroff Lab
Current research:
Prof. Rudroff’s research combines biocatalysis, synthetic organic chemistry, metabolic engineering, and systems biotechnology to design artificial multistep catalytic cascades in living organisms. His group investigates the interface of chemo- and biocatalysis, using retrosynthetic logic to construct de novo pathways composed of enzymes, organocatalysts, and metal catalysts. These “mini pathways” are introduced into heterologous hosts or examined in vitro to enable novel transformations, including the synthesis of fine chemicals, polyhydroxylated compounds, and natural fragrance and flavour molecules. His group has recently expanded into photobiocatalysis, exploring flavin activation under light and employing cyanobacteria as fully sustainable phototrophic chassis capable of using only CO₂, light, and inorganic nutrients for whole-cell catalysis. Additional research focuses include high-throughput droplet-based screening (FADS), enzyme redesign and stabilization, and light-driven oxidation strategies.
Research topics in a nutshell:
- Development of novel artificial enzyme cascades in E. coli and cyanobacteria.
- Creation of high-throughput functional assays for enzyme evolution.
- Application of photobiocatalytic strategies employing flavin activation under light.
- Biocatalytic production of fragrance aldehydes and pyrazine derivatives.
- New concepts for polyolefin degradation using light, oxygen, and photosensitizing proteins (doctoral project topics).
Florian Rudroff, born in Vienna, studied Technical Chemistry, with specialization in Organic Chemistry at the TU Wien. He conducted his diploma and PhD thesis in the group of Prof. Marko Mihovilovic and obtained his PhD in 2007 from TU Wien. The topic of the PhD thesis was located at the interface between Biology and Chemistry in the field of Biocatalysis. Afterwards he was awarded with an ’Erwin Schrödinger fellowship’ and went for a postdoctoral stay to the group of Prof. Uwe Sauer at ETH Zurich. His research was mainly conducted to Molecular Systems Biology (metabolomics and fluxomics) and specifically to the rapid metabolic and TOR signalling responses in Saccharomyces cerevisiae upon different nitrogen input signals.
In 2011, he returned to the TU Wien and started his independent scientific career in the field of Systems Biocatalysis. Since December 2017, he became Assistant Professor and finished his Habilitation in the field of Bioorganic Chemistry at the Institute of Applied Synthetic Chemistry, TU Wien. In 2020, he was appointed as Assoc. Professor and group leader of the Bioorganic Synthetic Chemistry Group (BSC) at the TU Wien. Since 2018 he is leader of the local branch (Vienna, lower Austria, Burgenland) of the Austrian Chemical Society (GÖCH).
His main research interests are located in the field of enzyme cascade catalysis, photoredox biocatalysis (e.g, CO2 utilization by cyanobacteria), protein engineering, the development of high throughput platforms (e.g. fluorescence adsorption droplet sorting, FADS) and organic synthesis.

GreenChemForCE
GreenChemForCE
Project official webpage: https://www.interreg-central.eu/projects/greenchemforce/
Project description:
- Challenge: Unsustainable chemical industry practices and poor public perception in Central Europe.
- Objective: Reduce environmental impact and revitalize the local chemical sector.
- Innovation: Implement advanced, sustainable, and circular economy processes.
- Outputs: Green production methods, industry training, and public awareness initiatives.
- Approach: Transnational collaboration across academia and industry.
- Cooperation: Shared expertise fosters sustainable regional improvements.
- Vision: Return of chemical production to Europe with eco-friendly practices.
Circular Bioengineering
Circular Bioengineering – Powering the Circular Bioeconomy
Project Website: https://www.circularbioengineering.at/
Project description:
- Objective: Revolutionize production and life cycles of goods through bio-based materials and zero-waste processes.
- Approach: Cross-disciplinary research combining molecular biosciences, biotechnology, and bioprocess assessment.
- Innovation: Develop technology-integrated biosystems (e.g., enzymes, cells) for sustainable, low-energy, and eco-friendly production.
- Sustainability: Focus on bioeconomy principles—minimal substance and energy use, zero waste, and environmental consideration.
- Impact: Train future scientists, engage stakeholders, and foster public awareness to advance the bioeconomy.
- Collaboration: Supported by five partner universities led by BOKU University, alongside the universities of Graz, Vienna, and their technical counterparts.
- Outreach: Serve the scientific community and stakeholders through research, education, and dissemination activities.
Pyrazine synthesis
Pyrazine synthesis – A greener way for our daily flavor and smell
Project description:
- Biocatalytic retrosynthesis for the production of heterocycles
- Biocatalytic synthesis of flavour and fragrance compounds via different enzymatic cascades
- Comparison of chemical & enzymatic routes
- FADS-based (fluorscence activated droplet sorting) enzyme-assay development
- Biomimicking of chemical flavour production starting from sugar beet molasses
BioZone
Bioinspired alkene cleavage – BioZone
The aim of this project is to develop a biological (enzyme-assisted) method for splitting carbon / carbon double bonds without the use of the dangerous ‘ozone’. The so-called ozonolysis is a widely used method for the production of so-called “carbonyl compounds”. Such carbon / carbon double bond containing compounds can be starting materials to make such as flavor compounds like vanillin or fragrances like Lilial. Ozonolyis is mainly used on a laboratory scale. The reason for this is the toxicity of ozone and the danger of the intermediate products, which are not safe: Some of them have already caused industrial reactors to explode. We want to find and investigate a safe alternative with enzymes that can split C = C double bonds at room temperature, in water and by means of oxygen. We use a high-throughput detection method that is specifically designed for the detection of aldehydes (carbonyl compounds) in order to examine different protein sequences for their ability to split C = C double bonds. This detection method is highly sensitive, completely independent of structure, easy to use and extremely specific for aldehydes. With this detection method in combination with a special technique, it is possible to examine up to a million protein sequences in a day. It is thus possible to examine known enzymes for their substrate diversity and new enzymes for their activity in a very short time, as quantitatively as possible. After suitable enzymes have been found, we will use them for the production of potential new active pharmaceutical ingredients or flavorings.
Cyanobacteria
Cyanobacteria – Photo-Biocatalysis goes green and blue
Recently, several studies have proven the potential of cyanobacteria as whole-cell biocatalysts for biotransformations. Compared to heterotrophic hosts, cyanobacteria show unique advantages thanks to their photoautotrophic metabolism. Their ability to use light as energy and CO2 as carbon source promises a truly sustainable production platform. Moreover, their photoautotrophic metabolism offers an encouraging source of reducing power, which makes them attractive for redox-based biotechnological purposes. To exploit the full potential of these whole-cell biocatalysts, cyanobacterial cells must be considered in their entirety.
Project description:
- Expression of redox enzymes in cyanobacteria
- Comparison of different cyanobacteria
- Biocatalysis – single step and in a cascade fashion
- Systems biology of cyanobacteria
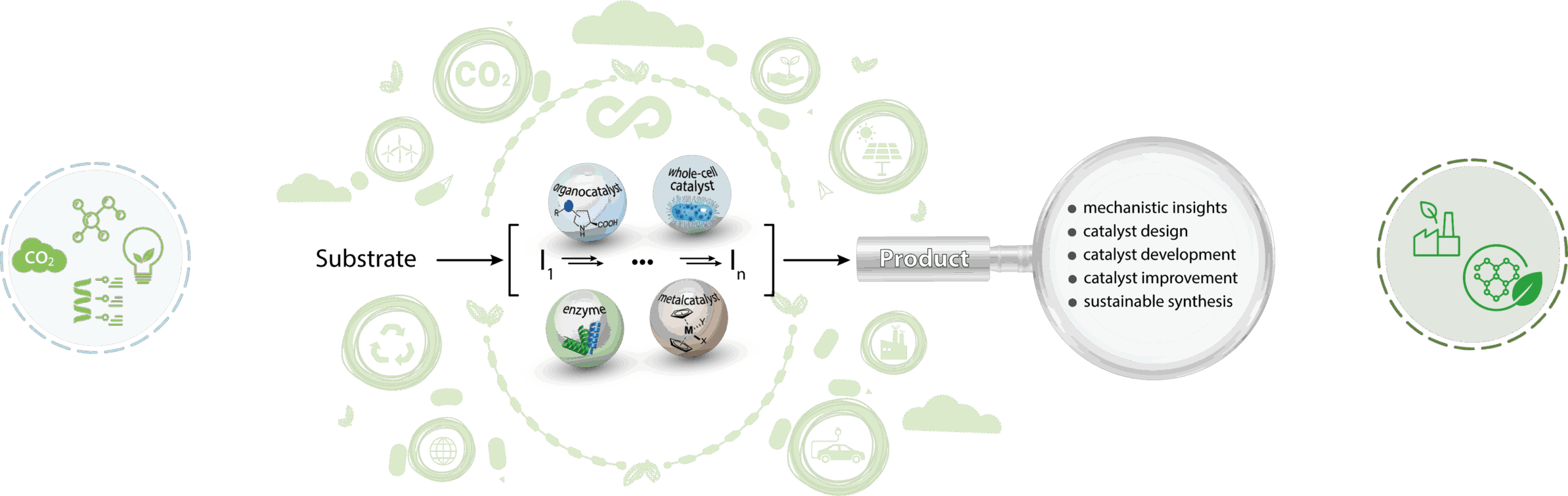
Enzyme cascades & Chemo/Biocatalysis
Enzyme cascades & Chemo/Biocatalysis
Project description:
- Development of novel strategies for the synthesis of high-value compounds by retrosynthesis
- Combination of chemo and biocatalysis
- Multi-step catalysis in vivo & in vitro
- Flux optimization – on transcriptional and translational level
- Protein engineering

Florian Rudroff
Univ.Prof. Dipl.-Ing. Dr.techn.
Connect
- +43 (1) 58801 163618
- +43 664 605 887 137
- florian.rudroff@tuwien.ac.at


